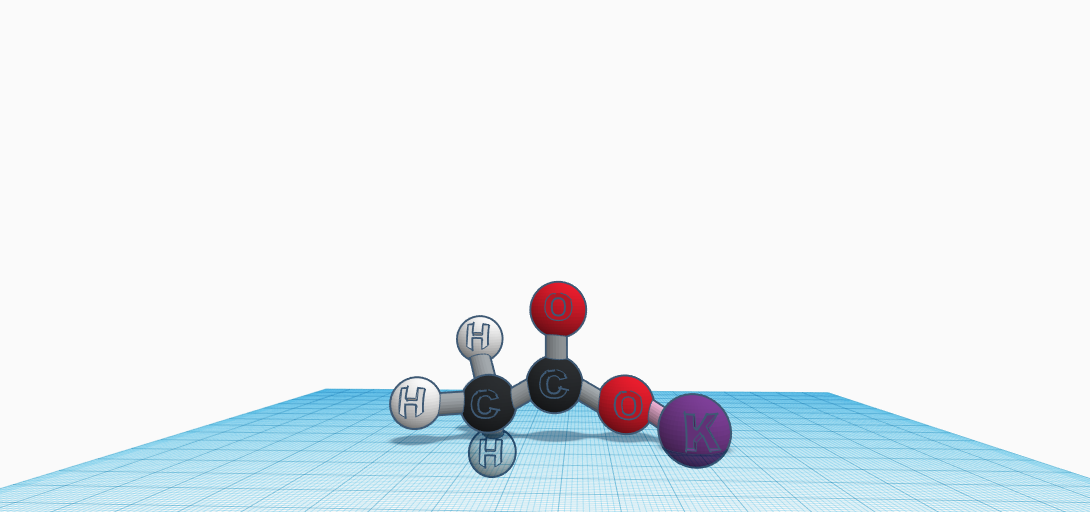
Potassium acetate is a pure white, crystal-like, deliquescent solid. It is seen as a powder in solid form and a clear substance when a liquid. It’s the potassium salt of acetic acid. The chemical has a density of 1.57 g/cm3. The molar mass of this chemical is 98.15 g/mol. This can be soluble in water, alcohol, and liquid ammonia. The solubility in water is 216.7 g/100 mL (0.1 degrees Celcius). Its melting point is 292 degrees Celcius. Potassium acetate’s compound or molecular composition consists of two carbon atoms, three hydrogen atoms, two oxygen atoms, and one potassium atom, or CH3COOK. The role that potassium acetate has is to be a food acidity regulator. You come in contact with potassium acetate every day. Walking in a grocery store and seeing Propel, Gatorade, or Powerade, are all examples of beverages that may have potassium acetate dissolved in the solution. It is known to replenish electrolytes that you need to hydrate your body, balance sugars, and raise your blood pressure. They give more energy and potassium acetate happens to help your electrolytes. Potassium acetate can also be used in de-icer. Just like how road salt prevents the formation of ice, so can potassium acetate with its acidic properties. It is used on airport runways because it offers the advantage of being less aggressive on soils and less corrosive than other de-icers. Lastly, another purpose is to use potassium acetate as a food preservative.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org