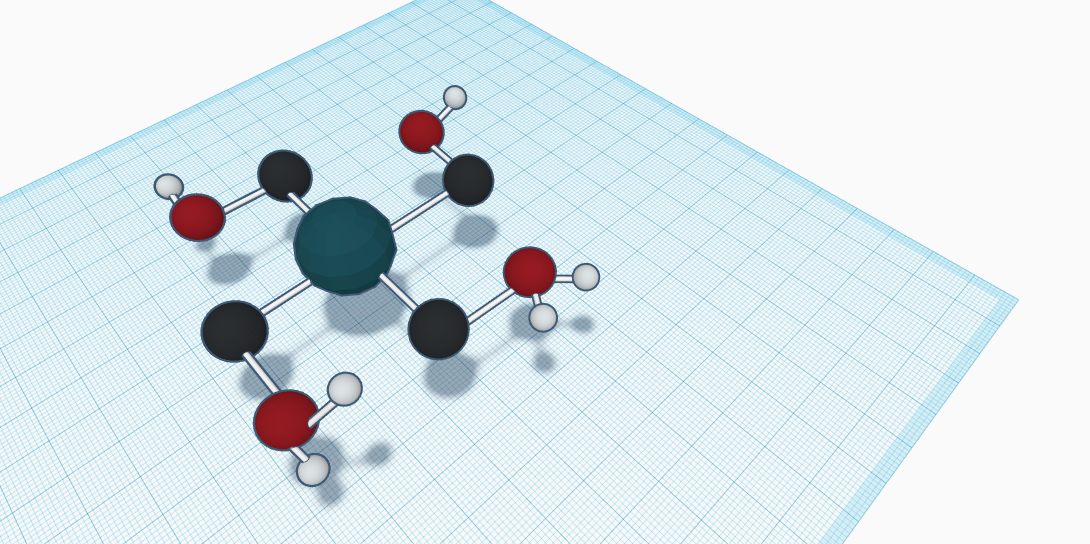
Cobalt has many compounds, so the first long compound that was found was Cobalt(ii) Acetate. Cobalt(ii) Acetate is a pink powdery acid. The formula is Co(CH3COO)2. Cobalt(ii) Acetate is a cobalt salt. This substance is soluble in water, dilute acids, pentyl acetate (tetrahydrate), and alcohol. The appearance is pink crystals intense red crystals. The melting point is 140 degrees celsius. The odor or smell, smells like vinegar. This substance is used for many things, and it is very important. This molecule has 4 different atoms, 3 plains, one up one in the middle and one down, it is all grouped, and to show the atoms are different I colored them. The navy blue atom is cobalt, black is for carbon, red is for oxygen, and white is for hydrogen. They are all different sized atoms and they create cobalt (ii) acetate. “It is also used as a catalyst for oxidation and esterification.” Which means it speeds up the reaction. This molecule has a density of 1.7 and it also has an appearance of a Pale pink to red to violet crystals or powder. The rounded mass is 177 and Cobalt Acetate is a moderately water soluble crystalline Cobalt source that decomposes to Cobalt oxide on heating. This molecule has elements of carbon, oxygen, hydrogen and mainly cobalt. Cobalt (ii) acetate is also known as Acetic acid; bis(acetato)cobalt; Cobalt(2+) acetate; Cobalt diacetate; Cobalt, ethanoic acid; CAS 5931-89-5, and cobalt salt. There are also more synonyms for Cobalt (ii) acetate.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org