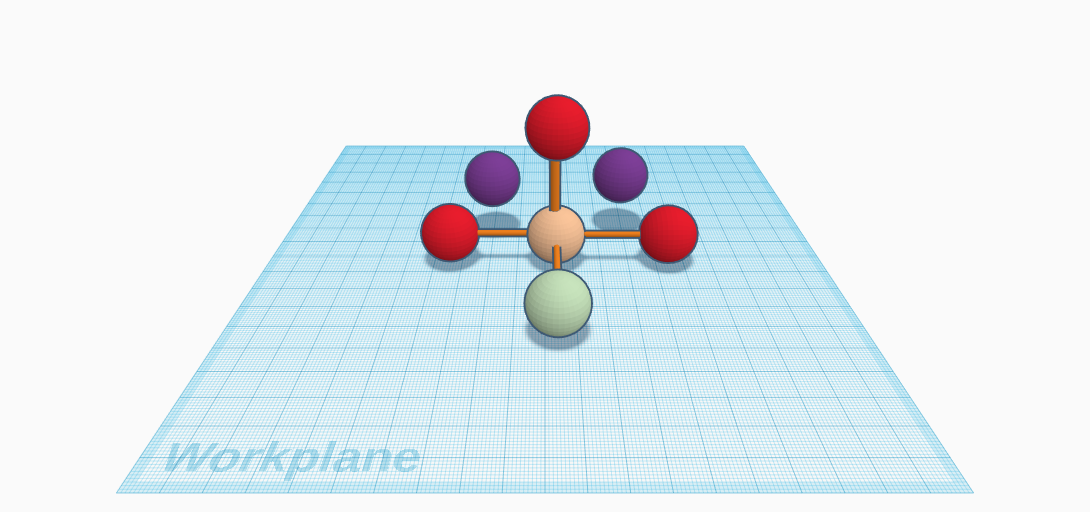
The chosen compound is Sodium Monofluorophosphate. It is also known as MFP. This compound is one of the many compounds associated with Fluorine. It was discovered by Willy Lange in 1929 at the University of Berlin. Lange was trying to make monofluorophosphoric acid when he discovered MFP. MFP has no color or odor. It is usually in the form of a white powder. It is also soluble in water. Its solubility in water is 25g/100mL. However, it is insoluble in ethanol, or alcohol. MFP melts at 625°C (898 K). The compound for MFP is Na2PO3F. There are two Protactinium atoms, one Phosphorus atom, three Oxygen atoms, and one Fluorine atom. The two Protactinium atoms are not bonded to the molecule. Sodium Monofluorophosphate is made from combining Sodium Metaphosphate and Sodium Fluoride. MFP is an inorganic compound. This is generally because it does not contain a carbon atom.
MPF is in a salt used in most toothpastes. It helps protect tooth enamel from cavities and makes the enamel stronger. MFP is sometimes preferred in toothpaste rather than straight fluoride because it has less of an aftertaste. It is usually a substitute for sodium fluoride in children's toothpaste because it is less “toxic”. MFP is sometimes used in medications that treat osteoporosis, which is a disease that weakens the bones. It is also used in water fluoridation. Water fluoridation puts fluoride in water supplies to prevent tooth decay.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org