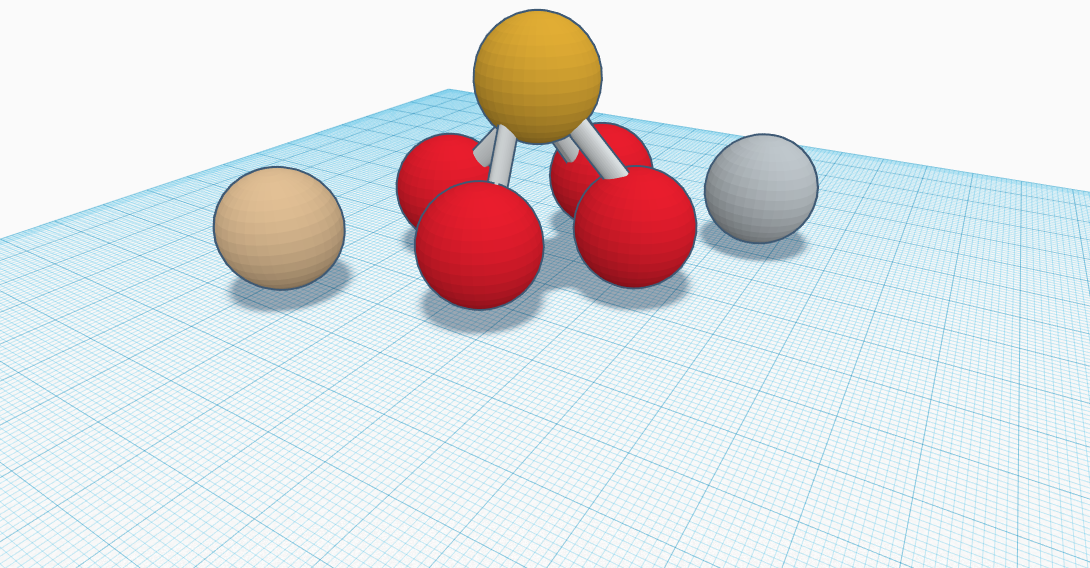
My compound is called Lithium Iron Phosphate. Lithium Iron Phosphate is written as LiFePO4 in its chemical formula. Lithium Iron Phosphate consist of one lithium atom, one iron atom, phosphorus atom, and four oxygen atoms. One of the oxygen atoms have two bonds while the other three only have one connecting them to the phosphorus atom. Lithium Iron Phosphate is a material that can be gray, red-grey, brown or black solid that is insoluble in water. Lithium Iron Phosphate is most commonly used to create ion batteries which are placed in power tools, vehicles, and solar panel installations. It is commonly called lfp and is also used to make educational laptops such as chromebooks. Due to the positive charges of both iron and lithium cause them to circle around the negative charges of the four oxygen and one phosphorus in the middle. It’s molar mass of the mass divided by amount of material or its in this case is one hundred fifty seven and seven thousands five hundred sevenths. It’s exact mass however is one hundred fifty seven and nine hundred and four thousandths . It’s melting point is three hundred degrees celsius. Lithium Iron Phosphate does not have a boiling point. Lithium Iron Phosphate is usually in the form of a powder but only in its standard state of twenty five degrees celsius.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org