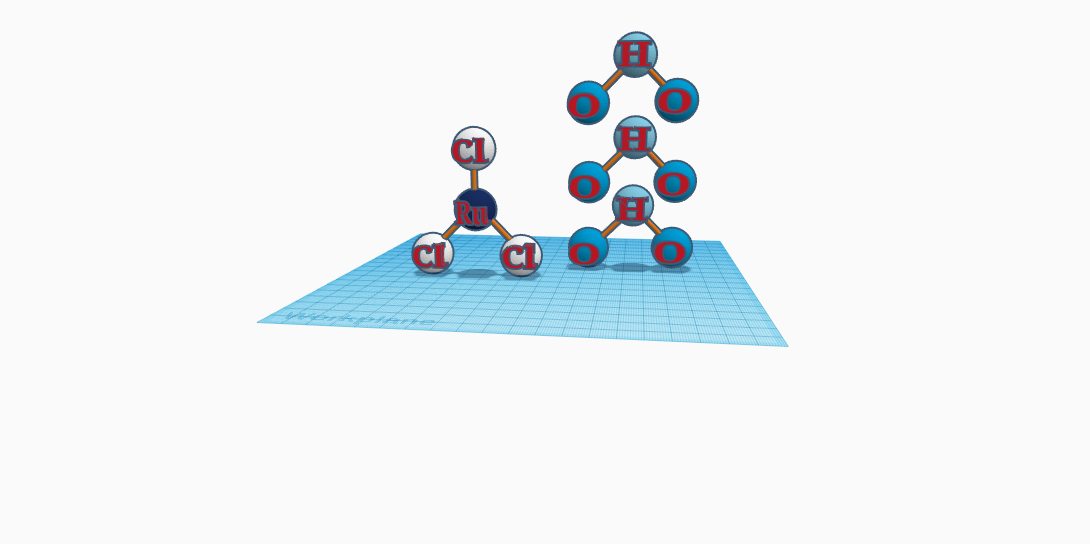
Known for being used to prepare a nanoparticulate ruthenium-aluminum oxyhydroxide catalyst for the efficient dehydrogenation arylmethyl alcohols to the corresponding aldehydes, Ruthenium Chloride Trihydrate is also a product that catalyzes the synthesis of two-ethyl-three-methylquinolines from primary aromatic amines and triallylamine. A Ruthenium Chloride Trihydrate molecule is made up of one Ruthenium atom connecting to three Chloride atoms, making the Ruthenium Chloride. Close to the Ruthenium Chloride, is the Trihydrate, containing three water molecules- three Hydrogen atoms and six Oxygen atoms. Although there are four molecules, when combined, they make one molecule- Ruthenium Chloride Trihydrate. Its molecular weight is 261.465 g/mol (grams per molecule). Ruthenium Chloride Trihydrate doesn’t have a common name, for the compound isn’t used in everyday lives. Ruthenium is also used in catalysts for ammonia and acetic acid products, along with being used in solar cells, which turn light energy into electrical energy. Ruthenium is also one of the most effective hardeners for platinum and palladium, and is alloyed with these metals to make electrical contacts for severe wear resistance, so it’s no wonder they use it with chlorine to prepare nanoparticulate ruthenium-aluminum oxyhydroxide catalysts for dehydrogenation arylmethyl alcohols to corresponding aldehydes. Put simple, ruthenium chloride trihydrate is a pretty great conductor.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org