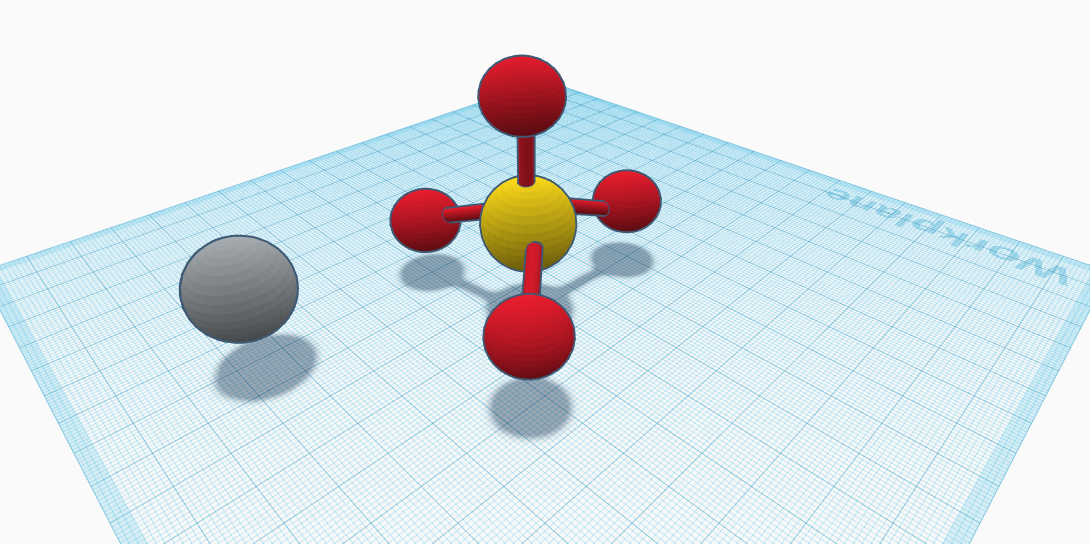
The compound copper sulfate has a formula of CuSO4. In the molecule, there is 1 copper atom, 1 sulfur atom, and 4 oxygen atoms. The sulfur atom is in the center of the molecule. The 4 oxygen atoms surround the sulfur. The copper atom is separate from the rest of the molecule. Copper sulfate is an electric blue crystal. The compound has no smell, and is toxic. It is not safe to work with. It could cause severe irritation to the eyes, mouth, and nose. If a large amount of copper sulfate is swallowed, it causes vomiting, nausea, damage to body tissue, damage to blood cells, damage to the liver, kidneys, and more. In extreme cases of exposure, death could occur. Copper sulfate has a density of 3.6 g/cm³. It has a melting point of 230°F, and is soluble in water. It has a molar mass of 159.609 g/mol. Copper sulfate has many uses. It is a fungicide, meaning it is used as a chemical that kills fungus. It is used to control fungus on fruits and vegetables. It kills bacteria, algae, and bugs as well. Copper sulfate can be found in fertilizers to protect the food, and pesticide products to keep away unwanted bugs. It is also found in chimney and wood stove cleaners. On wooden stoves, the wood burns and then a residue called creosote is left behind. This is because certain chemicals from the wood burning don’t vaporize. If copper sulfate is applied, the creosote burns off. As one can see, copper sulfate has various uses in the world.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org