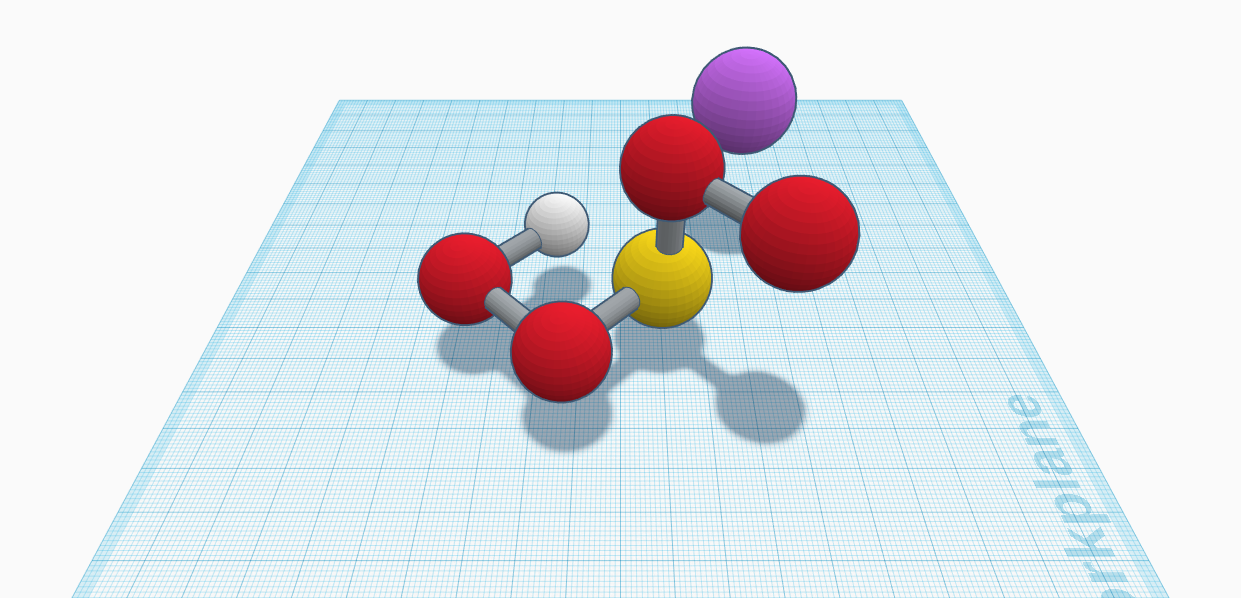
The molecule sodium hydrogen sulfate, also known as sodium bisulfate, is the sodium salt of the bisulfate anion with a molecular formula NaHSO4. This has one sodium, hydrogen and sulfur atom and four oxygen atom, sodium being the largest to sulfur, oxygen, and finally, hydrogen being the smallest. The oxygen atoms are red, the hydrogen atoms are white, the sulfur atoms are yellow, and the sodium atoms are violet, including other alkali metals. This molecule has a total of seven atoms. It also has many synonyms including bisulfate of soda, sodium acid sulfate, sodium hydrosulfate, etc. The appearance of this molecule is white and is a solid. It also has a molar mass of 120.06 g/mol (anhydrous) or 138.07 g/mol (monohydrate), and it has a density of 2.742 grams per cubic centimeter (anhydrous) or 1.8 grams per cubic centimeter (monohydrate). Their melting point is 315°C (anhydrous) or 58.5°C (monohydrate), and its boiling point is 315°C (monohydrate). It is soluble in water as 28.5 g/100 mL (25°C) and 100 g/100 mL (100°C), but it’s insoluble in ammonia. It is revealed in our world in many different uses. It is used in foods, swimming pools and hot tubs, beverages, metal finishing, cleaning products, companion animal food, etc. This molecule is known to be safe and is approved for use in Australia, New Zealand, Canada, and Mexico. Its food grade is used in many varieties of food products, including beverages, dressings, sauces, and fillings. It is known that we eat this every day
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org