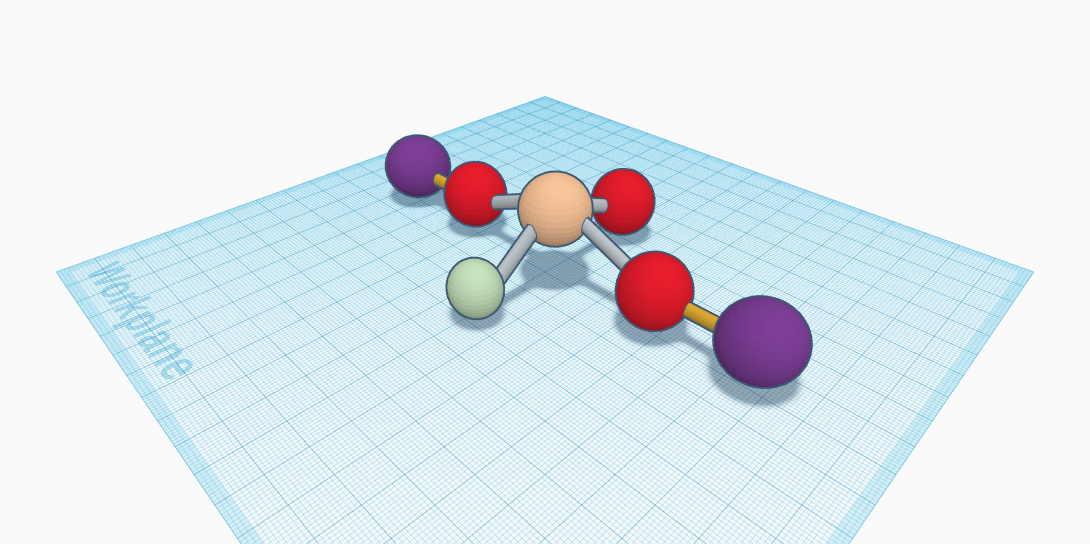
There are so many elements and compounds that make up our universe, but an important one is Sodium Monofluorophosphate. This is the compound of Na2PFO3. It is made of 2 Sodium, one phosphorous, one fluorine, and three oxygen. It is able to be found in everyday toothpaste. This compound is in the structure of having the sodium in the middle, the three oxygen and one fluorine connected to it, and then the two sodium on the outside, but aren't connected to the other atoms. It is odourless, colorless, and water soluble. Sodium Monofluorophosphate is also known commonly as a salt to most scientists and communities. Sodium monofluorophosphate is one of three types of fluoride recognized by the U.S. Food and Drug Administration to help prevent dental cavities in children, teens, and adults. Sodium monofluorophosphate in toothpaste safely and effectively helps to prevent tooth decay, when formulated correctly and used the correct way. Sodium monofluorophosphate is recognized by the United States Food and Drug Administration and the European Community as safe and effective as an anti-cavity agent in oral care products. There have been multiple tests with this compound, such as the one with rats. The results showed that the increase in bone thickness was marked higher in this compound then the other tested compound, sodium fluoride. This is a very important ingredient for everyday life, and the people of this earth are happy to have it.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org