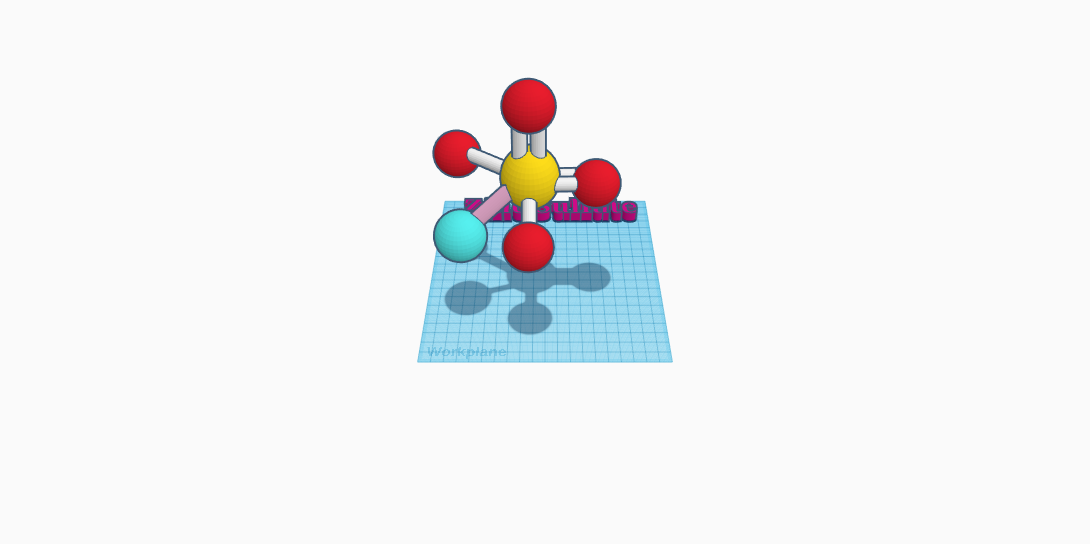
Zinc sulfate is a white crystal or powder. This compound consists of six atoms and three elements. This includes zinc which has an atom color of blue, sulphur which has an atom color of yellow, and oxygen which has an atom color of red. It has a density of 3.54 g/cm³ and is soluble in water. It’s formula is ZnSO4. Its melting point which is at 680 °C and has its boiling point at 740 °C. Its mass is 161.47 g/mol and remains odorless. Also known as white vitriol, it’s used in toothpaste, as a pesticide to kill mosses, and in medications like eye drops. The zinc atom within the molecule isn’t actually technically connected, but will be in my entry. The molecule has a sulphur atom in the middle, with four oxygen atoms surrounding it. Two oxygen atoms have two bonds connected to them, while the oxygen atoms have only one. The zinc atom is to the side but is not connected to the rest of the molecule. Zinc is a natural mineral. Its important for tissue development and health. Zinc sulfate helps treat depleted zinc levels. As a powder, zinc sulfate is an eye irritant. Zinc sulfate can be toxic, depending on the levels of zinc sulfate. Certain side effects of it can be is heartburn, nausea, and stomach upsets. Zinc sulfate is a naturally occuring substance in water, food, plants, and soils. It will crystallise with water as an aqueous solution into a heptahydrate. All in all, zinc sulfate has multiple uses in everyday items such as toothpaste, medications, and a pesticide killer.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org