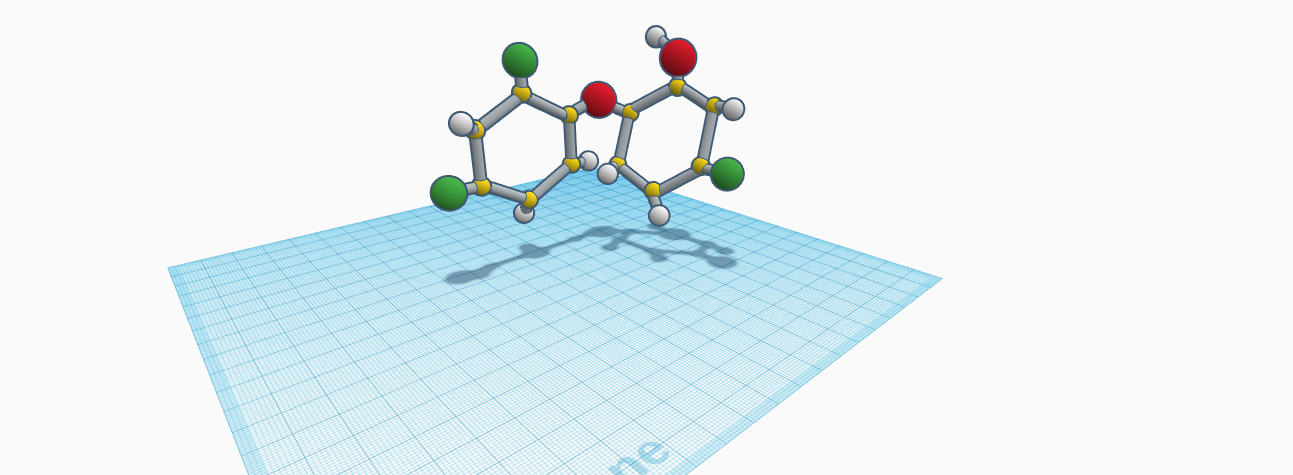
Triclosan is a very important substance in today's world. Without it, you can't have clean teeth, fresh breath, or even clean clothes or toys to play with. It is an antibacterial and antifungal agent. The molecular makeup of it is C13H8Cl5. There are a total of 26 atoms and 3 different elements. So, there are thirteen carbon atoms, eight hydrogen atoms, and five chlorine atoms. The molar mass of it is 289.54 g/mol. The molecular weight is 331.524 g/mol. The density of it is 1.49 grams per cubic centimeter. The boiling of it is 120 degrees celsius. The melting point and freezing point is 55 degrees celsius. Triclosan is a white powder. There are some risks of using it. It is used in many detergents, toothpastes, soaps, and deodorant. Humans have become so accustomed to using these products, but haven't looked into what is really in them. If very young children are exposed to it they are more likely to develop, allergies, asthma, and eczema. Clearly, this substance can be very harmful to young children. Not only is it used in the inside household things we use everyday, but it's also used in pesticides and other harmful things. If your in the kitchen, the playroom, the bathroom, or even just enjoying the outdoors, you are more than likely exposed to some form of it. Triclosan is made in many different factories and countries because it is so important. It has a faint aromatic odor. It is not very soluble in water, but is soluble in other substances such as caustic soda.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org