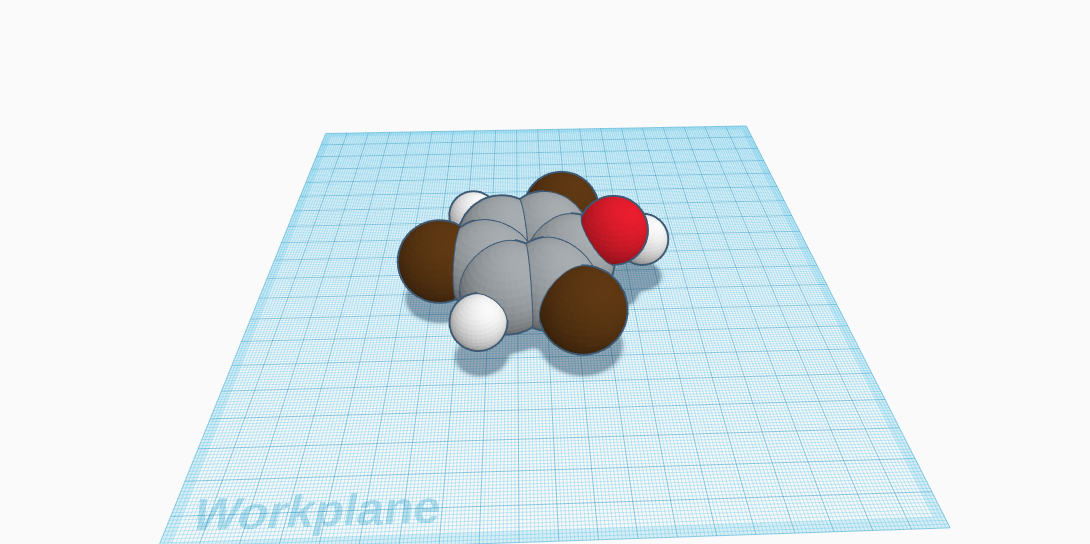
One compound of bromine is 2,4,6-Tribromophenol. Its formula is C6H3Br3O and it contains a total of 13 atoms, and 4 elements. There are carbon (6 atoms), hydrogen (3 atoms), bromine (3 atoms), and oxygen (1 atom). In the molecular structure, there is a circle of six carbon atoms in the middle. Every other carbon atom has one bromine atom connected to it, so three carbon atoms each have one bromine. Then one of the carbon atoms that doesn’t have a bromine atom connected to it, has a oxygen atom connected. The other two carbon atoms without an bromine nor a oxygen atom, have a hydrogen atom. Therefore, the two remaining carbon atoms have one hydrogen atom each, and the third hydrogen connects to the oxygen atom. 2,4,6-Tribromophenol is commonly used to kill dangerous fungi, preparation for flame retardants, and wood preservative. It's found in crustaceans and molluscs, but it's mostly industrially made. It's a flavor component of seafood, and usually in powder form. It looks like long, white crystals, smells like bromine, and feels soft. It can also have a slight brown color. Its water solubility is 71 mg/L (15 ºC). It's an organic compound (a compound that contains at least one carbon atom). However, it can irritate eyes and skin. It’s molecular weight is 330.8, vapor density is 11.4 (versus air), and regular density is 2.55. It’s boiling point is 282-290 ºC, and the melting point is 90-94 ºC. 2,4,6-Tribromophenol has many worldly uses and it very unique.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org