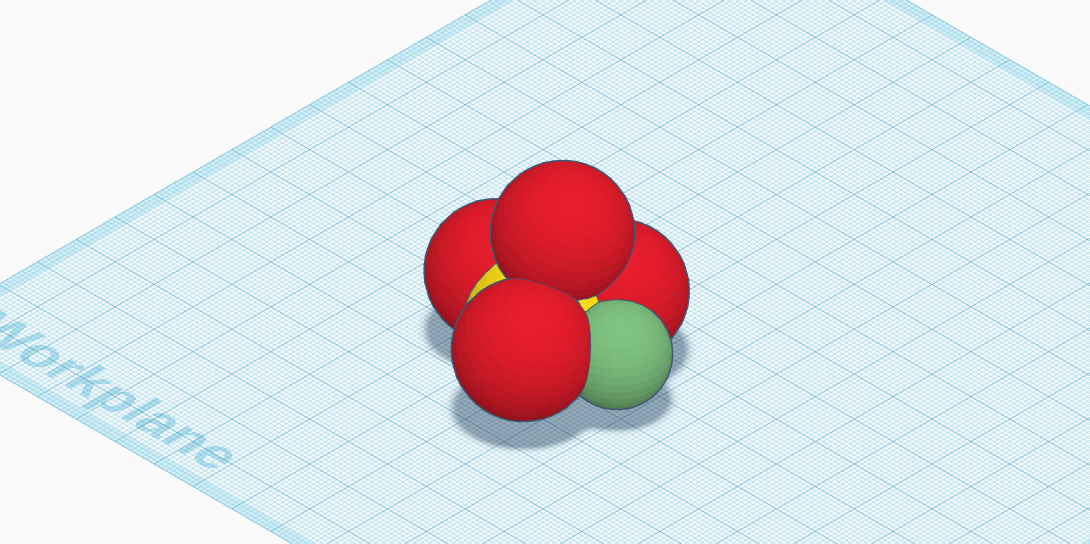
My molecule is nickel sulfate. It’s molecular composition is NiSO4. Nickel sulfate is a combination of nickel, sulfur, and oxygen. The molecule is made up of one atom of nickel, one atom of sulfur, and four atoms of oxygen. Its relevance in the real world is it is used for electroplating nickel on metal objects. The metal objects that are electroplated are used in daily life and many are household items. Some of them are kitchen utensils, scissors, needles, and even vacuum cleaners. Many people don’t realize it, but they interact with nickel sulfate everyday. Another relevance of nickel sulfate in the real world is it is used to create other nickel compounds. One property of nickel sulfate is its density, which is 3.68 grams per centimeter cubed. A second property of nickel sulfate is its boiling point. Its boiling point is 1,544 degrees fahrenheit. The third property of nickel sulfate is its melting point of 127.94 degrees fahrenheit. A fourth property of nickel sulfate is its molar mass, which is 154.75 grams per molecule. The fifth property of nickel sulfate is its appearance. Nickel sulfate is usually a greenish blue color. It is made up of tiny crystals, so it is a solid. A sixth property of nickel is its odor. Nickel sulfate doesn’t have a smell to it, so it is odorless. In conclusion, nickel sulfate is a molecule that many people interact with daily. Some properties of nickel sulfate are its density, boiling point, melting point, molar mass, appearance,and its odor.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org