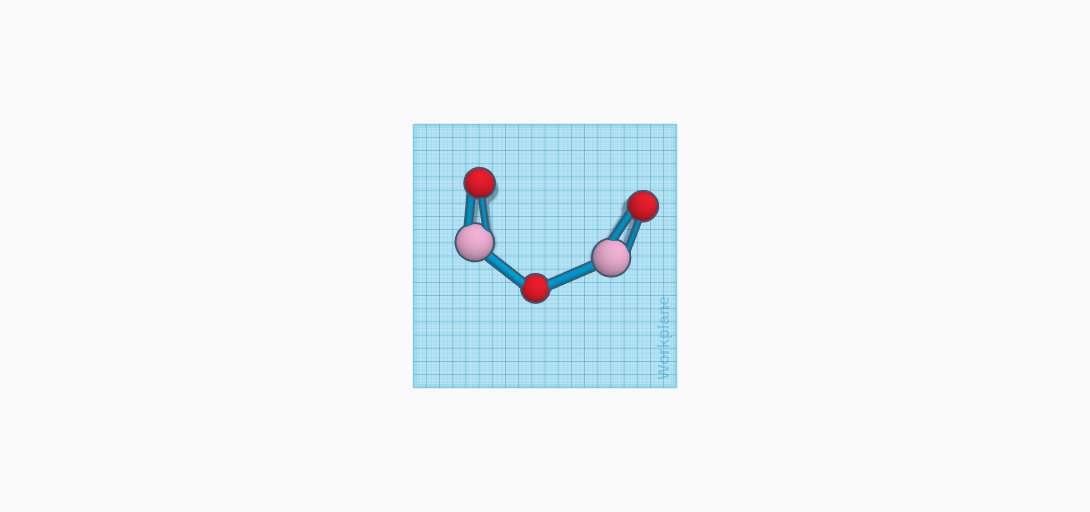
Erbium is used in many molecules. One molecule erbium is used in is erbium oxide. The compound for erbium oxide is Er2O3. In this compound, there are two atoms of erbium. There are also three oxygen atoms. Two bonds are attached to each of the two oxygen atoms. These oxygen bonds each connect to a different erbium atom. The erbium atoms both meet in the middle, attaching to another oxygen atom with one bond. It is not a complex molecule, yet erbium oxide is used in many products that are used in everyday life. Since erbium oxide is a pink color, it is used to make the common household item, sunglasses. The glass is tinted using erbium oxide which creates the most important part of the sunglasses. This protects users from the sun while also providing them with a stylish accessory. Another household item is vases. Once again, erbium oxide is used to tint the glass. Not only is it in household items, but it is used as a burnable neutron poison for nuclear fuel. The nuclear fuel is used to power vehicles that are used every day. The erbium oxide molecule itself looks like a crystalline powder. It is a heterogeneous mixture since the powder is pink. In addition, erbium oxide is not soluble, which means that it does not dissolve in water. This is beneficial when creating certain fuels. Erbium oxide has a density of 8.64 grams/cubic centimeters, and a boiling point of 3,290 degrees Celsius. The melting point is 2,344 degrees Celsius. Overall, erbium oxide is an important molecule.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org