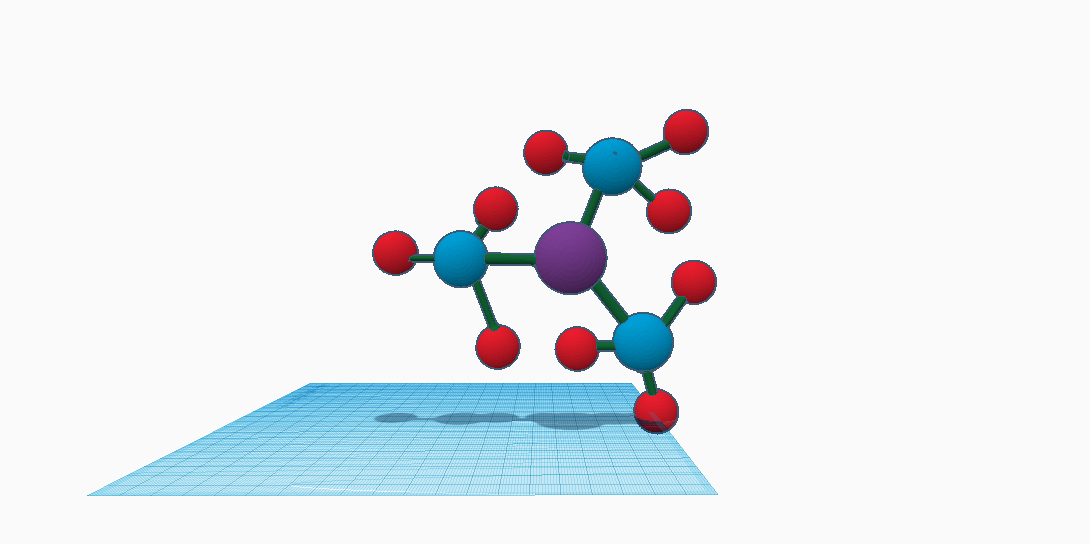
The Bismuth element has numerous compounds (molecular composition). One of the more popular ones happen to be Bismuth 3 Nitrate also written as Bi(NO3)3. It is the reaction between bismuth (3) and hydroxide (oxide and nitric acid). It is salt composed of bismuth in its cationic +3 oxidation state and nitrate anions. The pentahydrate is the most common solid form. It has the unusual property of being able to expand upon solidification which allows it to make castings for sprained/broken bones as well type metal alloys. It is most importantly used to manufacture low-melting alloys such as Wood’s metal, which is used in things like electrical fuses, automatic fire alarms, and sprinkler systems. Since it is salt composed, it can be used in the synthesis of other bismuth compounds and is available commercially. This is the only nitrate salt that is formed by a group 15 element. It can be prepared by the reaction of bismuth metal and concentrated nitric acid. One of its properties include the boiling point which is 75-80 degrees Celsius, but the melting point is 30 degrees Celsius. Another property is the molecular weight which is 485.07. The density is 2.83 and measured in grams/ cubic centimeters, so the density is actually 2.83g/cm cubed. The appearance is sometimes in a white crystal form, and it’s solubility in water is that it can and will decompose. There are two types of mass for Bismuth 3 Nitrate and that is the exact mass and monoisotopic mass is also 484.996676.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org