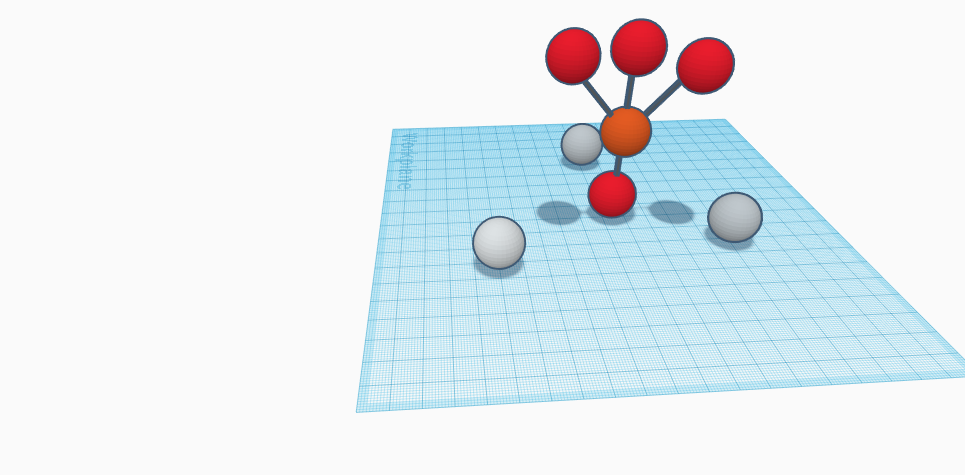
Silver phosphate or silver orthophosphate is a light sensitive substance and the molecular composition of silver phosphate is Ag3PO4. It is a solid yellow powder, water-insoluble chemical compound, and is moderately soluble in acid or solutions. Its solubility in water is 0.00065 g/100 mL, with a density of 6.37g/cm3 , melting point of 849°C, and the molar mass, the mass of a given substance divided by the amount of substance, of silver phosphate is 418.58 g/mol. It becomes opaque or discolors when impure and is formed as yellow precipitate by the reaction between a soluble silver compound, such as silver nitrate with a soluble orthophosphate. The ion phosphate at high temperature can suffer a reaction of condensation to form pyrophosphate, in those conditions it is also possible to form silver pyrophosphate when silver nitrate is added. Depending on the method of preparation different crystal forms of silver phosphate can be produced of the same lattice structure. It is used as a standard for determining the concentration of phosphates in solutions. It is also used as a staining of biological materials. Since silver phosphate is a light sensitive agent it is used in photography as emulsions. It is used as catalysts on some reactions and for medical purposes as astringent, bactericide, etc. Silver phosphate is also a potential material for incorporating silver ion antibacterial properties into materials. It can cause respiratory diseases, coughing and skin and eye irritation.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org