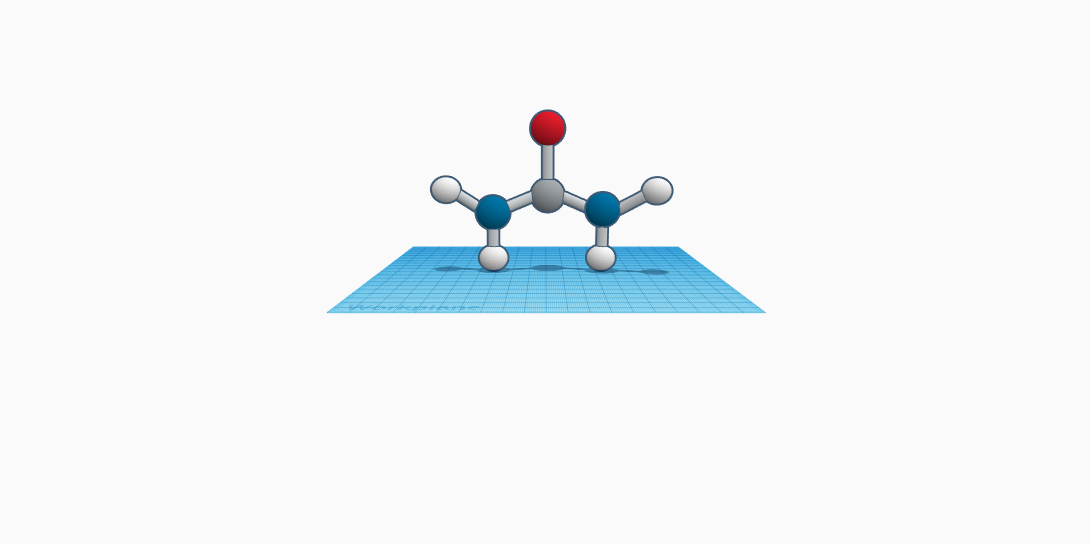
Urea was discovered in 1773 by Hilaire-Marin Rouelle. It is an organic compound with the formula CO(NH2)2 and is a waste product of living organisms. Urea, which is sometimes referred to as carbamide, is a solid compound that contains nitrogen. In urea, there is a carbonyl group attached to two amide groups. The amide group in urea contains nitrogen attached to two hydrogen atoms. It is a colorless, crystalline, and is odorless. At the boiling point it decomposes and melts at 271 degrees Fahrenheit. It has a density of 1.32 g/cm cubed and is highly soluble in water. The formula weight of the object is 60.6 pounds. This material occurs in the urine, blood, sweat, and milk of mammals. Humans carry 25 grams of urea. In the liver, the urea cycle breaks down excess amino acids into ammonia, then transforms ammonia into urea. The nitrogen in the amino acid becomes converted to ammonia. It is further converted by the liver into urea, which is less toxic than ammonia to the body. The kidneys will then filter out the urea from the circulation. After that, it will send it to the bladder along with water and excrete it in the form of urine. Urea is used to treat dry/rough skin and to treat nail problems. It is used to help remove dead tissue in some wounds to help wound healing. It is also used in agriculture as fertilizer and to feed animals and is the most used nitrogen fertilizer.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org