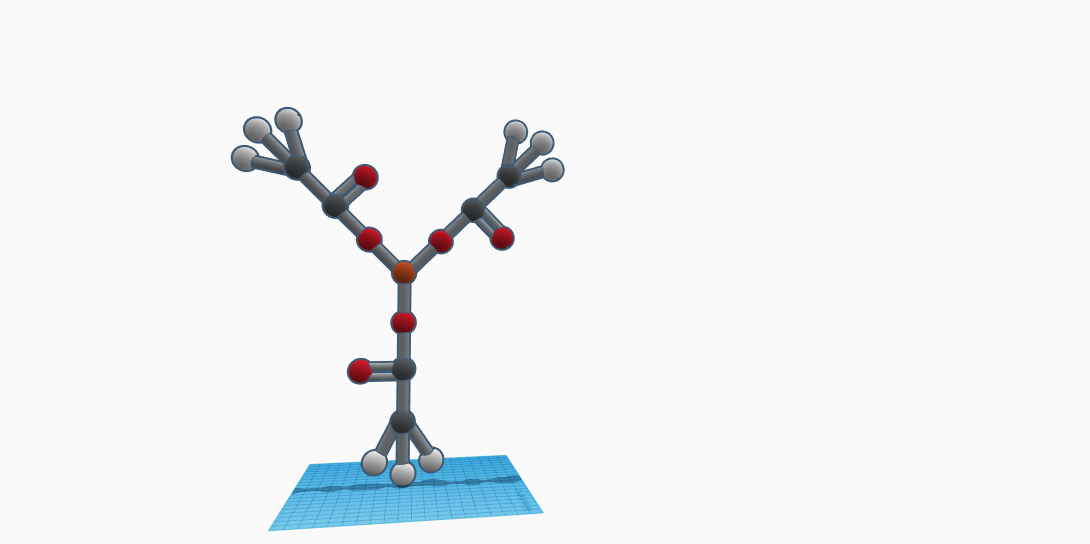
The molecule, Ferric Acetate or Iron Acetate or Iron(lll) triacetate, composes of 22 atoms and 4 elements. The compound is written as Fe(CH3COO)3. In this case, there is 1 iron, 6 carbon, 9 hydrogen, and 6 oxygen atoms. Notice that the elements carbon, hydrogen and oxygen are in parentheses with a subscript of 3. This is next to the one element, iron. So there's one iron atom (ferric) connected with three acetate groups. Each acetate group holds 2 carbon, 3 hydrogen, and 2 oxygen atoms. The iron atom at the center. The relevance of ferric acetate in our world is that it is used in the dyeing of cloth. Iron acetate can be prepared by heating iron, acetic acid, and in the presence of air. It was used in the 19th century to produce sprinkled effects on leather. It is currently used as a mordant for textile dyes and as a wood preservative for furniture by staining it. The properties of ferric acetate is that it is insoluble in water, but soluble in ethanol, which is alcohol. The color of iron acetate is mainly red-brown, or light brown, with a form of amorphous powder. The state of matter is a solid. The molecular weight for ferric acetate is 232.977 g/mol. The exact mass is 232.975 g/mol. Iron acetate is classified under the chemical and drug category. It is also under organic chemicals, and organometallic chemicals. It is part of iron compounds, ferric compounds. Iron acetate reacts chemically with the tannins in the wood; one coat is all it takes to give oak a dark cast.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org