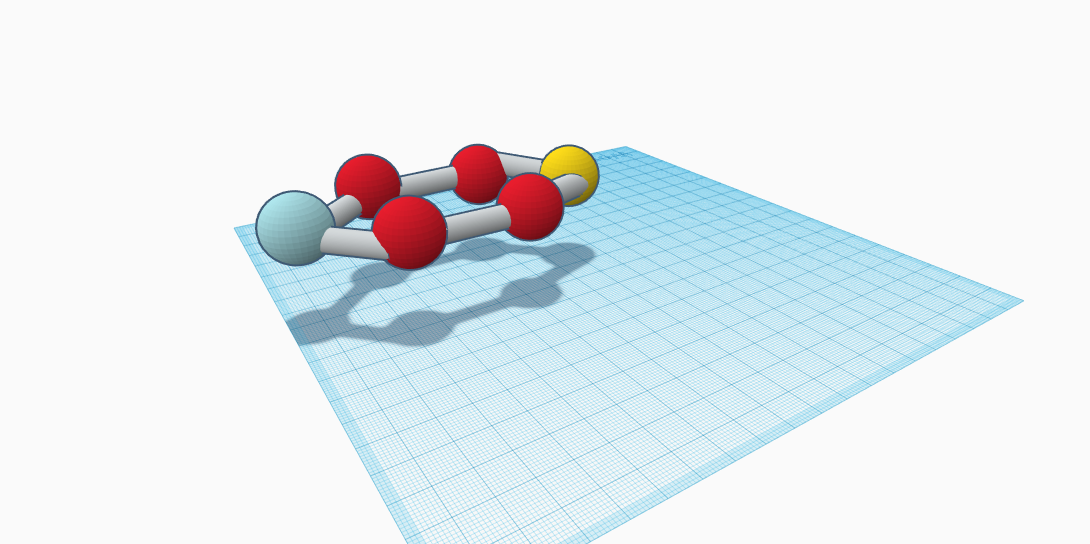
The element lead contributes to the compound Anglesite. The atomic symbol for lead is Pb. The molecule for this mineral is PbSO4. The composition for Anglesite would be lead sulfate. This molecule contains one lead atom, one atom of sulfur, and four oxygen atoms. It is a secondary lead mineral which means that it contains the element lead, but not too much of it. Anglesite, which is a rock, is tinted gray-black, with crystals sticking out. The crystals could be orange, yellow, transparent, blue, or a colorless white. They can also be transparent or even translucent. Since it is a rock it is a mixture of many different minerals. Furthemore, Anglesite is a homogeneous mixture because the minerals that are combined cannot be easily separated. It is also brittle and in the group of sulfates. Anglesite is used in many household items. A few examples of these items would be batteries, which are used everyday in toys and other things, plumbing which is used in kitchens and bathrooms, glass, which is on windows, tv’s, phones, etc., sound absorber, which is used in speakers and Bluetooths. And last, but not least, paint pigment, which is paint which is in the paint painted on walls or in the paint children use. This mineral is naturally found in the following places: Wales, England, Scotland, Austria, Slovenia, Germany, Sardinia, Russia, Tunisia, Morocco, Namibia, The United States, Mexico, and Australia.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org