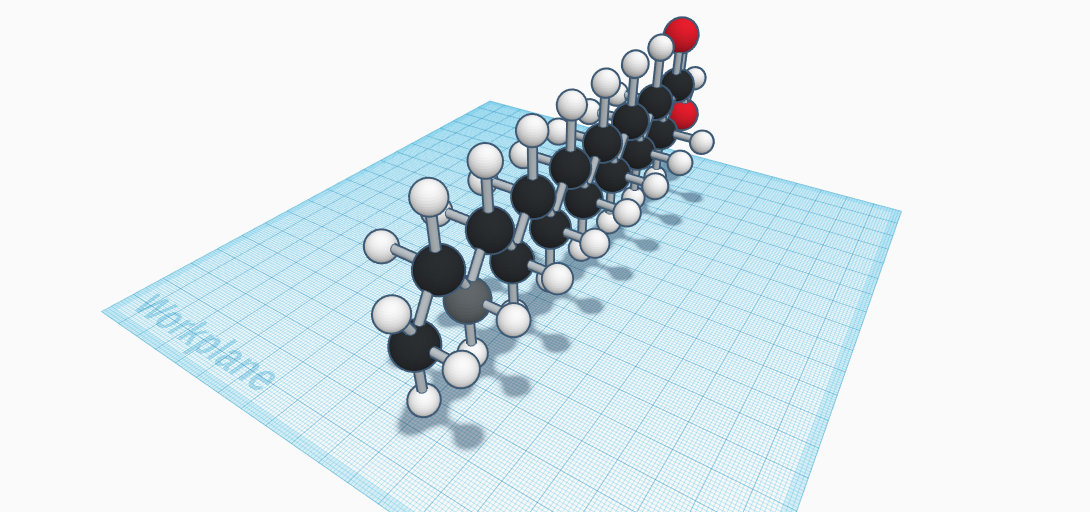
Palmitic Acid is a chemical compound with the chemical formula of C16H32O2. This compound consists of sixteen atoms of Carbon, thirty-two Hydrogen atoms, and two oxygen atoms to create a total of fifty atoms. Palmitic Acid is one the most common saturated fatty acid found in animals, plants and microorganisms. Also known as hexadecanoic acid, it can be found as a major component of the oil in fruit palms, or palm oil. There are also salts and esters of palmitic acid called Palmitates. The palmitate anion is the viewed for of palmitic acid at a physiologic pH (7.4). It was discovered by Edmond Frémy in 1840. He discovered this in saponified palm oil. This remains as the main industrial route for its production, with the fats, or triglycerides, in the palm oil being hydrolysed by a high water temperature. This resulted in a mixture that was partially distilled to produce the product purely. This chemical compound is naturally produced by a various range of other plants and organisms, usually at levels that are lower. The density of palmitic acid is about 0.85 g/cu cm. The boiling point of this compound is 351.5 degrees Celsius, and its melting point is 61.8 degrees Celsius. It is usually found in the form of a solid, with white crystalline scales. It is odorless and has a molecular weight of 256.43 g/mol. Its is presented in our world naturally in butter, cheese, milk, and meat. It also presented in cocoa butter, soybean oil, and sunflower oil.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org