Bracelets, necklaces, earrings are only a few of the jewelry that uses diamond imitation. In diamond imitation, Galliumgarnet is used. This helps with the making of fake stone for fashion. Luckily it can be manufactured in various colors that are popular in diamonds today. Diamond imitation is used all around the world and is very common and inexpensive compared to the real thing.
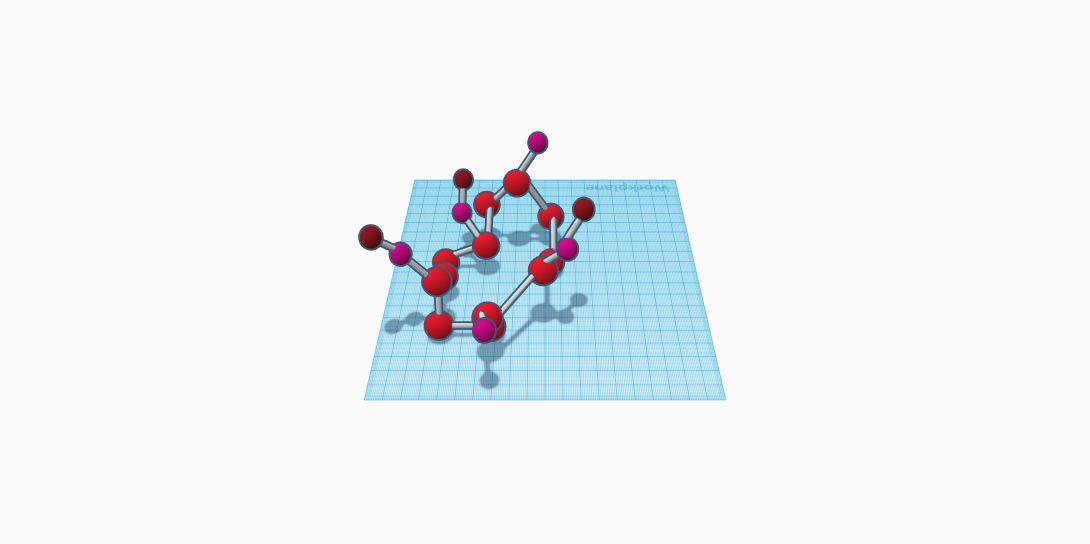
In the molecule of Galliumgarnet there are 20 atoms with three different elements. Each different color represents a different element. First off, the red atom represents oxygen. There are 12 different oxygen atoms in my molecule, along with 3 maroon Gadolinium atoms, and 5 pink Gallium atoms. Thus, these 3 elements make up the compound of Gd3Ga5O12. The melting point of Galliumgarnet is 1730 degrees Celsius and the boiling point is unknown. Also, the density of Galliumgarnet ranges between 7.08-7.1 g/cm3. The color of Galliumgarnet can range from being light brown/yellow, orange, or blue. It can exist as a solid or liquid upon slight change in temperature.
The use of diamond imitation, is to make a real diamond look fake. The most popular diamond substitute is the cubic zirconia. It is significantly more affordable than a real diamond, especially for those who may be on a budget. Many of the imitations look like the real thing. One can design it with colored diamonds like pink or chocolate and accents, giving it the charm of the real diamond. Galliumgarnet is very useful everywhere!
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org