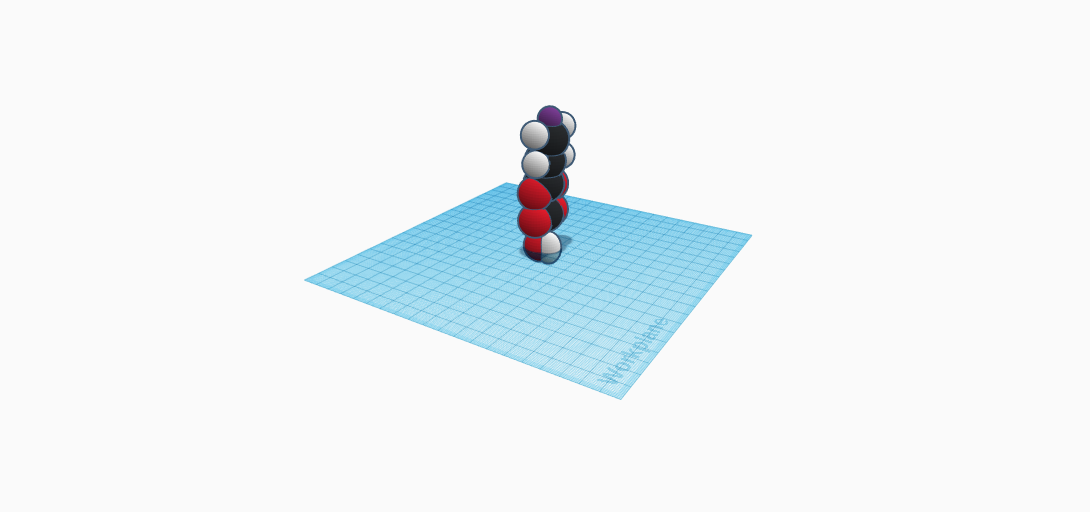
The compound Potassium Bitartrate has a few different names such as: Potassium Hydrogen Tartrate and Potassium Acid Tartrate, but it is most commonly known as cream of tartar. The formula of it is KC4H5O6, and was discovered by a Swedish chemist Karl Wilhelm Scheele in 1770. It is white, orderless, and a crystalline powder. The compound has a density of 1.05 g/cm3, a molar mass of 188.177 g/mol (mass of a given substance divided by the amount of that substance), and a chunky texture. It is used in cooking/baking and in cleaning. More specifically, it is used in baking/cooking is used: for stabilizing white eggs, stabilizing whipped cream, maintaining whipped creams texture and volume, thickening cake, preventing sugar from crystallizing, and reducing discoloration of boiled vegetables. It can also be mixed with icing for gingerbread houses and other frosting to prevent sugar from crystallizing. In cleaning, it can be mixed with lemon juice or vinegar to make a paste solution for cleaning metals. It can also just be mixed with plain water and used as a solution for removing light stains. It is also known to be one of the safest chemicals for general use. Moving on, it is made from the same process as for centuries. Which is when wine lees (leftover solid material after grapes have been crushed to make wine) are treated with hot water,and then let to evaporate. After, Potassium bitartrate crystals form, and then are removed and purified.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org