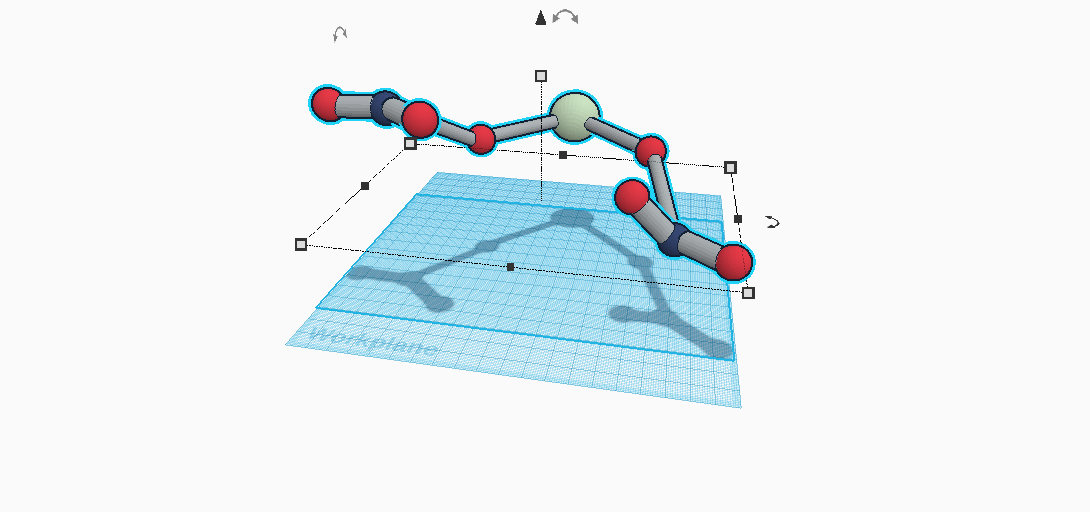
Calcium Nitrate is made up of three different elements and contains a total of nine atoms. This compound’s formula is Ca(NO3)2. There is one calcium atom, two nitrogen atoms, and there are six oxygen atoms in calcium nitrate. There are four long bonds and four short bonds holding these atoms together to make a molecule. It is used in many different day-to-day uses. Want to plant some flowers in a garden? It's is in fertilizer. Just fell and got bruised? It's is in ice packs. Wanting a sidewalk next to the road to walk? It's is in concrete, too. Calcium Nitrate can either be in a tetrahydrate form or an anhydrous form. As a tetrahydrate, its physical properties include the following: mass, density, color, melting point, boiling point, acidity, and crystal structure. Its color is actually nothing at all; it’s a colorless solid. It's mass comes to about 236.15 g/mol. This compound’s density is calculated to be about 1.896 g/cm3. It’s melting point and boiling point weighs in at 42.7 degrees celsius and 132 degrees celsius, respectively. Calcium Nitrate’s acidity is a 6.0, as well. Finally comes crystal structure, which is a monoclinic structure. These physical properties are actually different when Calcium Nitrate is in an anhydrous form, though. By just listing them off, we see that its properties as an anhydrous would be: 164.088 g/mol mass, 2.504 g/cm3 density, 561 degrees celsius melting point, 6.0 acidity, and a cubic crystal structure.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org