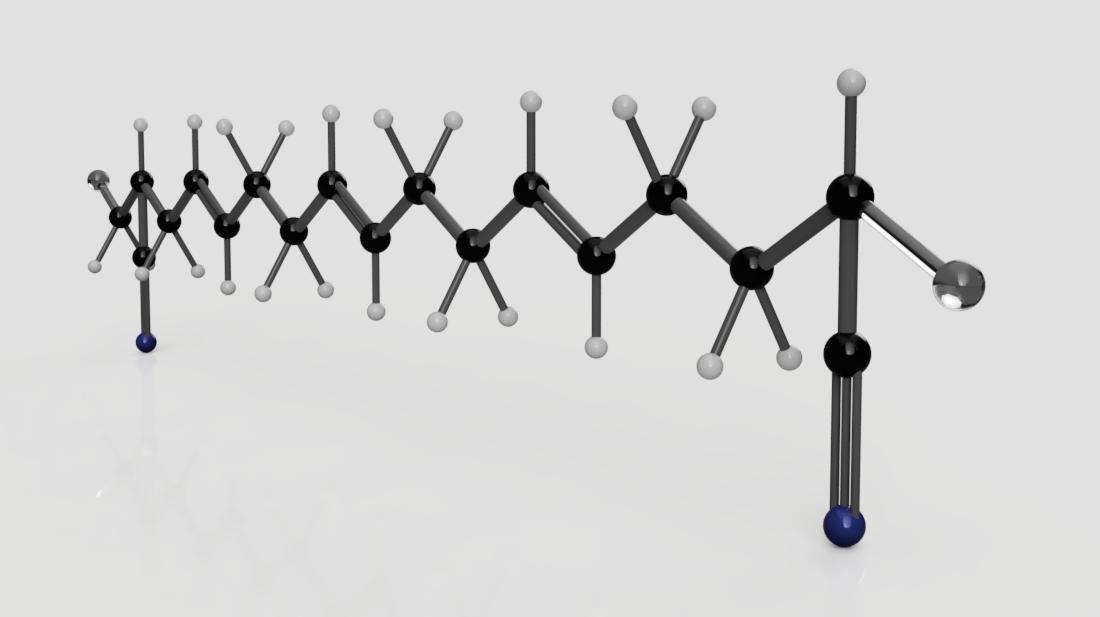
Nitrile is a family of unsaturated copolymers of two distinct kinds of monomers, butadiene monomers (1,2-butadiene and 1,3-butadiene) and 2-propenenitrile. Based on the desired properties of the final product, the manufacturer can add nitrile monomers; adding more will increase its resistance to oils while lowering its flexibility and decreasing the amount will do the opposite. The molecule I’ve made here is one example of how this elastomer can be constructed. The one use of nitrile rubber that we’ve all heard of is in disposable medical and lab gloves, but it’s also everywhere on the industrial scene; the automotive and aeronautical industries use it to make O-rings, gaskets, oil seals, hoses, synthetic leather, adhesives, transmission belts and more. Overall, nitrile has high abrasion resistance, tear resistance, and resistance to non-polar solvents, water, and oil; however, it has reduced flame, ozone, sunlight, and weather resistance.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org