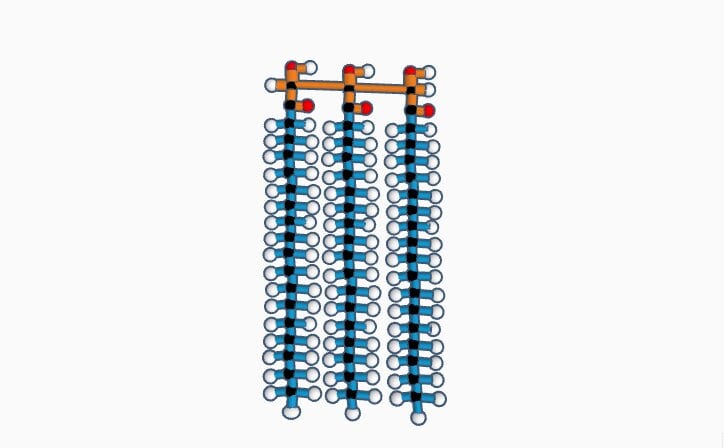
I chose stearin, a type of triglyceride or fat, because it is important to wash hands with soap in this pandemic. Stearin is used in the making of soap where it is combined with sodium hydroxide. When soap hits the phospholipid bilayer membrane on the coronavirus, it destroys the membrane. Stearin is made of 3 stearic acids and 1 glycerol. Stearic acid has the formula C18H36O2. Glycerol has the formula C3H8O3. C3H8O3 + 3C18H36O2 - 3H2O= C57H110O6 which is stearin. A water molecule is removed in the process of combining each stearic acid with glycerol. This process is called Hydrolysis. At room temperature, stearin is a white powder. Its molar mass is 891.501 g/mol-1. Its IUPAC name is Propane-1,2,3-triyl trioctadecanoate. Stearin is mainly found in palm trees. Michel Eugène Chevreul discovered it in the 1820’s. It boils at 813°C, melts at 54℃ and has a density of 0.862 g/cm3.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org