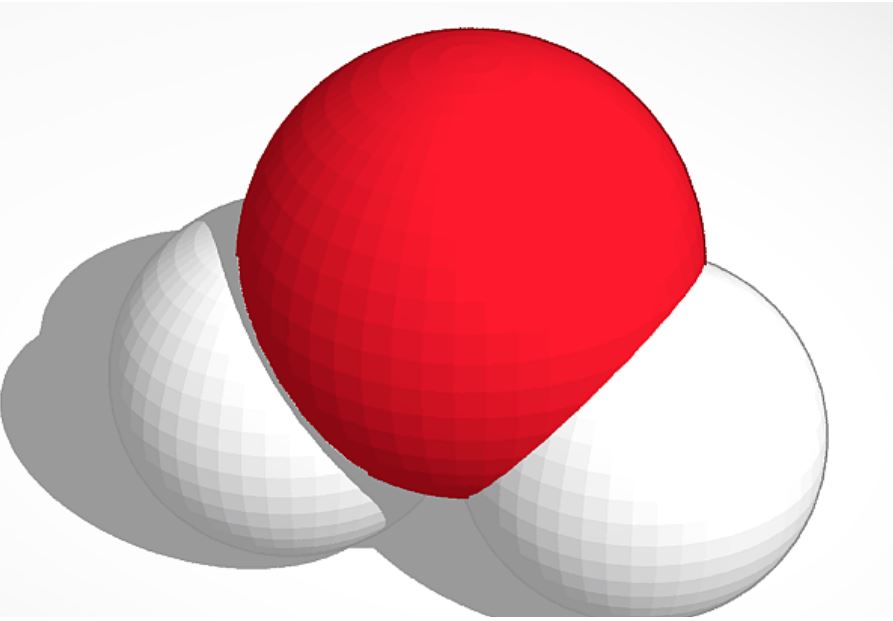
The molecule H2O, also known as dihydrogen monoxide or water, is a very important molecule in our lives. All living beings use water for their survival. Even 60% of our body is composed of water.
H2O has many scientific properties. This molecule consists of three atoms altogether and has two hydrogen atoms and one oxygen atom. The molecule uses a covalent bond to keep itself together. A hydrogen atom needs one more electron and an oxygen atom requires two more electrons to complete its outer shell. When an oxygen atom does a covalent bond with two hydrogen atoms, the oxygen atom and the hydrogen atoms are sharing an electron each between each other and completes all outer shells. When H2O molecules bond, they use a hydrogen bond. The H2O molecules in the solid form are tightly-packed; in liquid they are flexible; and in the gas form, they move freely.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org