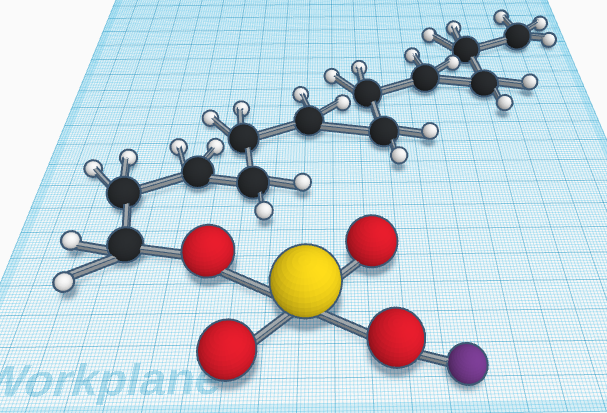
This molecule consists of 12 carbon atoms, 25 hydrogen atoms, 4 oxygen atoms, one sulfur atom, and one sodium atom. This is a common ingredient in soap and other cleaners. It breaks up oil by forming microscopic spheres of the oil and surrounds these oil balls with the soap molecules. The reason that it can dissolve oil in water is because it’s polar on one end and nonpolar on the other side. This molecule is especially important because this past year there has been COVID and we have had to make sure to wash our hands especially well to get rid of germs. Cleaning and disinfections our hands and surfaces is important because we need to stay clean. Sodium lauryl sulfate is important, but it is especially important because of covid.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org