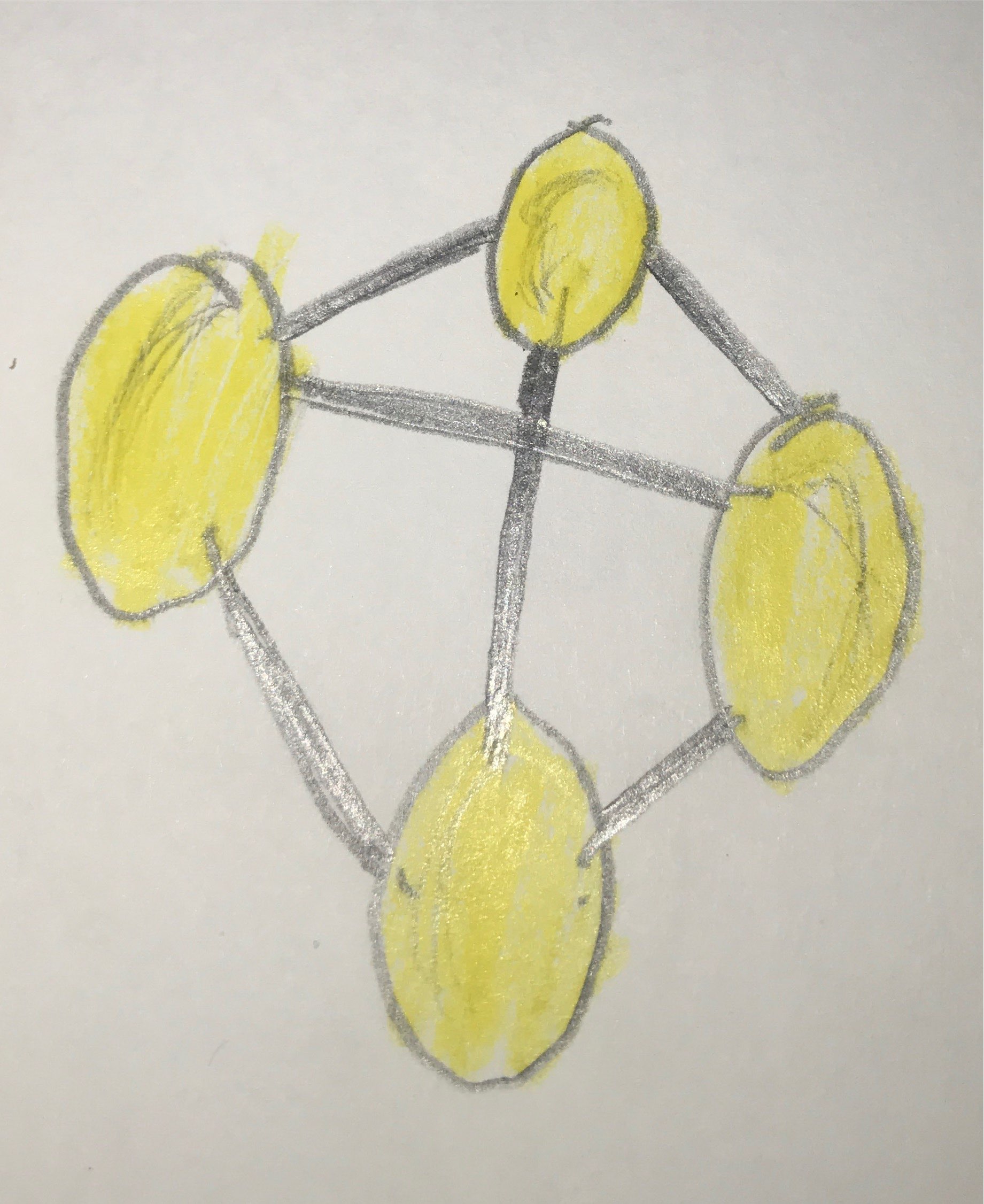
White phosphorous is the most volatile, unstable, toxic allotrope. Each atom in a tetrahedral P4 molecule is connected to the other atoms by a single bond. It is essential for life because it is a part of RNA, DNA, ATP, and all cell membranes. Under light and heat, it can turn into red phosphorous, which is a polymer. Phosphoric acids are used as fertilizers. They are also used in detergents. It is also part of calcium phosphate salts that harden bones.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org