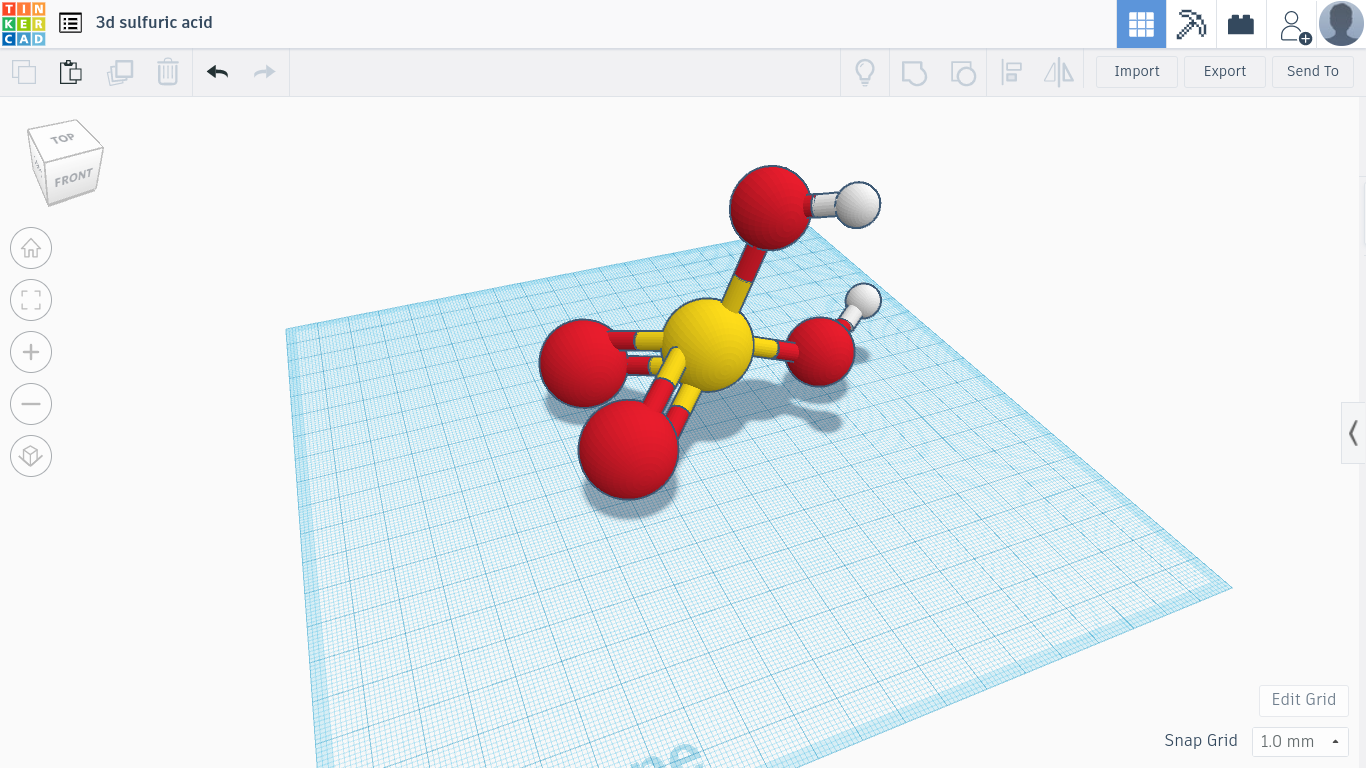
The sulfuric acid molecule is a colorless liquid. The formula for this molecule is H2O4S. This means that sulfuric acid is made up of 2 hydrogen atoms,4 oxygen atoms and a sulfur atom. In industry,sulfuric acid is created when sulfur dioxide is mixed with water. This is the formula: SO3(sulfur dioxide)+H2O(water)=H2O4S(sulfuric acid). Sulfuric acid damages the skin of a person that touches it due to it’s high acidity. Sulfuric acid was found by chemist John Glauber in the late 17th century. Sulfuric acid is mainly used to grow plants. In fertilizers,sulfur is added to the soil of the plants. The soil changes the sulfur to become sulfuric acid. Then,the sulfuric acid lowers the pH(phosphorus) of plants. High pH hampers the plant’s ability to take nutrients. Sulfuric acid lowers the pH level so the plant could grow.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org