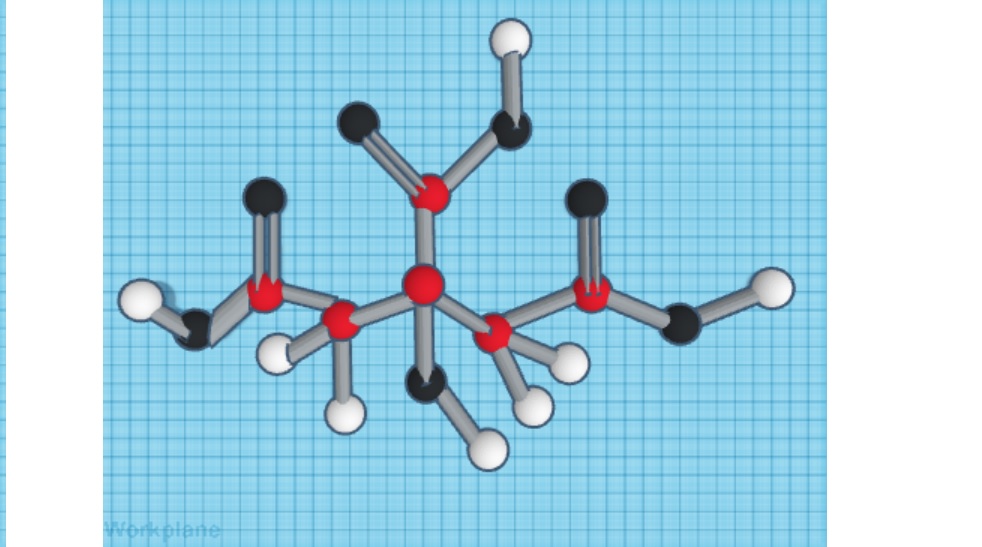
The scientific name of Citric Acid is hydroxypropane tricarboxylic acid. Citric Acid is a weak acid usually found in citrus fruits and drinks. It is odorless and colorless, highly soluble in water, with a density of 1.66 g/mL, a melting point of 153 °C, and a boiling point of 175 °C. The molecular formula of Citric Acid is C₆H₈O, that is, it contains 6 Carbon (red), 8 Hydrogen (white), and 7 Oxygen (black) molecules. In everyday life daily, it is used to add taste to citric fruits like lemon, lime, and oranges. Citric acid is also essential to the Citric Acid cycle during cellular metabolism. The Citric Acid Cycle (also known as the Krebs Cycle), is the series of different chemical reactions used by animals that can survive with oxygen to release stored energy from the oxidation of acetyl-CoA derived from different carbohydrates.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org