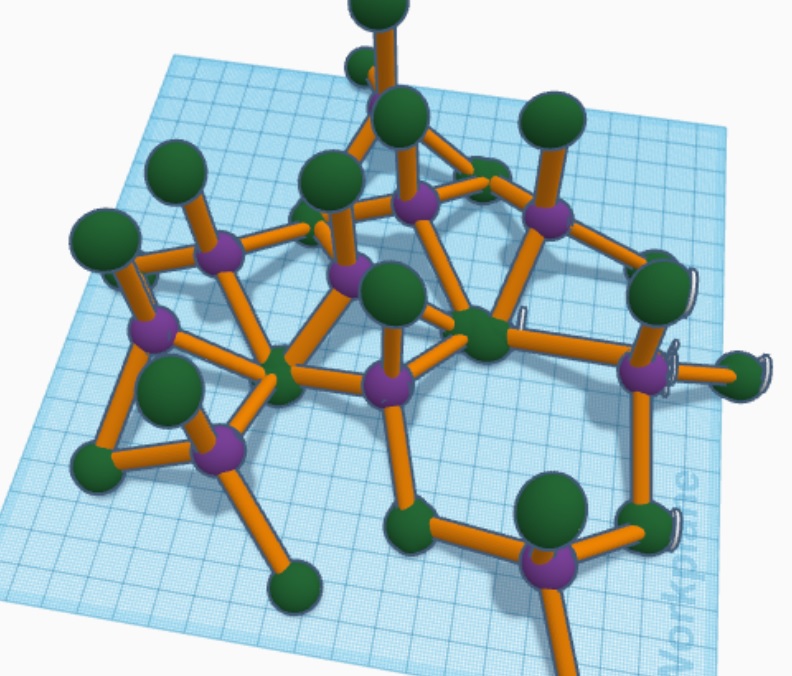
My molecule is silicon dioxide, also known as silica.
Silica’s one of the main components in glass. It is used in the most common type of glass we see every day: soda-lime-silica glass.
Silica’s in the shape of a tetrahedron. Its composition is silica attached to 4 oxygens. The adjacent tetrahedrons share oxygens. In my molecule the purple sphere in the middle is the silica and the 4 green spheres around it are oxygens.
Glass’s made by heating sand at about 1,760°C until it turns into a liquid. Then, you rapidly cool it.
Glass is an amorphous solid because when you rapidly cool the liquid glass it is turning into an object which does not have the crystalline structure of a solid nor is it a liquid. My molecule shows how it is in an amorphous structure because the structure’s not in a pattern and is randomly placed.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org