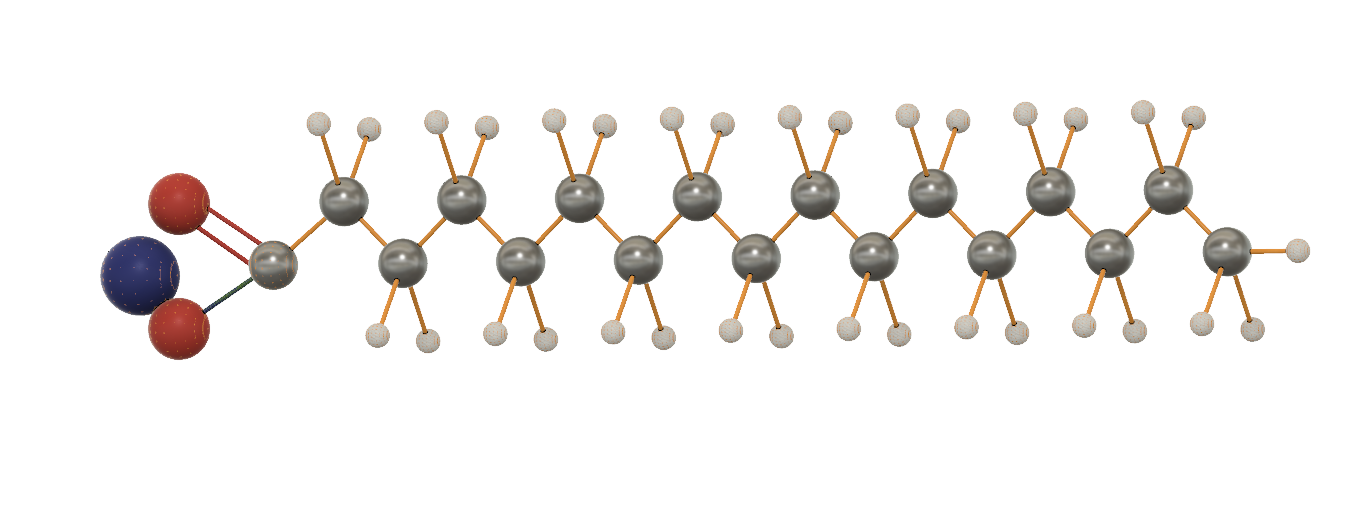
Soap molecules consist of Carbon, Hydrogen, Oxygen, and Sodium atoms. My molecule’s formula is C16H33COONa but there can be other variations of soap based on which acids are used to make it. Soap molecules have a hydrophilic (water loving) head and a wiggly hydrophobic (water hating) tail.
When soap is mixed with water, soap molecules make ball-shaped clusters called micelles. The hydrophobic tails stay inside the micelles and trap germs, oil etc. As water is poured, the hydrophilic part gets attracted to water and takes the soap and the trapped germs away with it.
I made a soap molecule because soap saves lives. Soap has helped end Cholera and other pandemics in the past. It is playing a big part in controlling the spread of COVID-19. It keeps our body, home, and surroundings clean and hygienic.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org