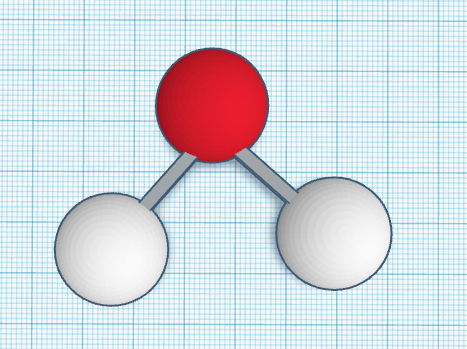
A water molecule, H2O, contains three atoms - two Hydrogen and one Oxygen. The atoms are bonded by sharing electrons, which pull them together. The bond is called a polar covalent bond. Water is less dense as a solid than as a liquid. As water freezes, the molecules form a different structure that spaces the molecules further apart than in liquid water. This means that ice is less dense than liquid water, which is why it stays on top of the water. This property is important, because it ensures that lakes, ponds, and oceans that freeze don’t freeze underneath the surface of the water. This protects all the animals underneath the icy surface. Water is important because without it humans would dehydrate and die, so it is essential to human survival. I chose to make my water molecule because it was simple but very interesting.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org