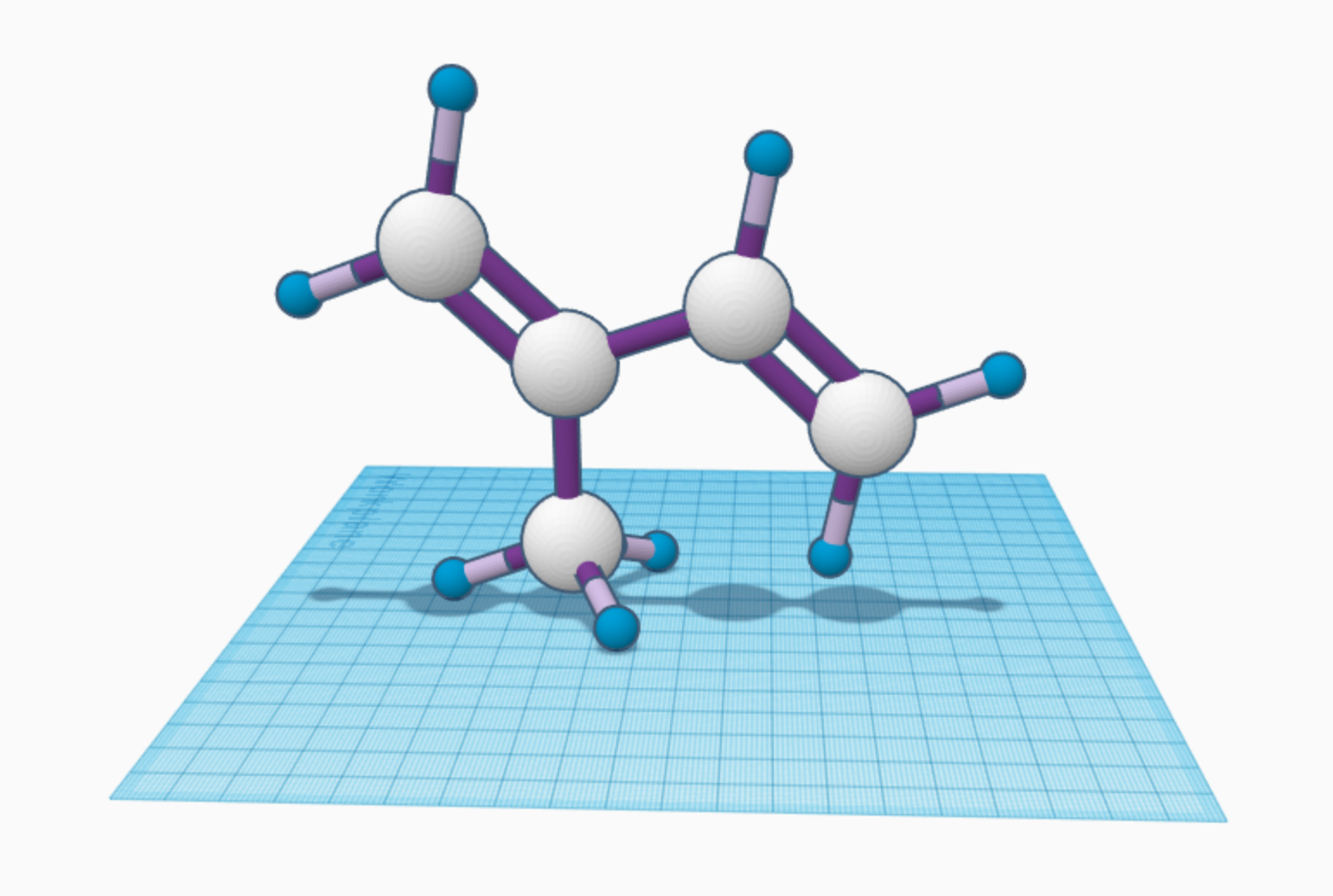
I chose the compound molecule isoprene. Its formula is C5H8, which means it contains 5 carbon and 8 hydrogen molecules. Natural isoprene is a very flammable, colorless liquid found in plants and animals (even humans!). Too much isoprene can be harmful to the environment and it is a carcinogen for people, but as a polymer it is the basis of natural rubber. Rubber has many uses because it can be hard or soft. I use rubber every day: in bands that tie my hair back, on the bottom of my shoes so I won’t slip, to erase mistakes at school, and in my bicycle tires to create friction with the road. Doctors and nurses also use rubber in their gloves to keep safe during the pandemic.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org