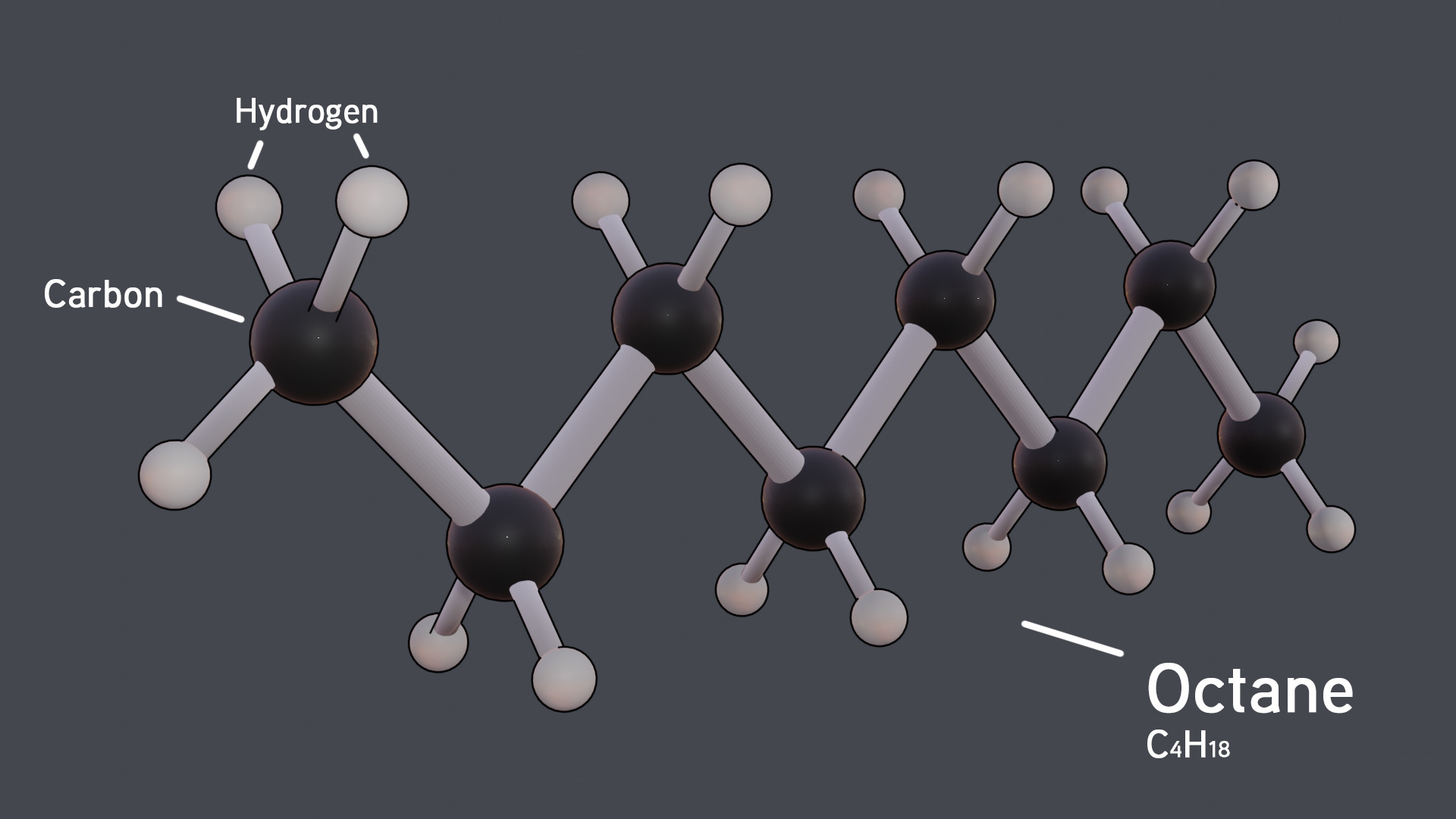
N-octane (C8H18) is a hydrocarbon that is commonly added to gasoline, which many people use to commute every day. It raises the pressure required to ignite gasoline, which prevents it from igniting early in a combustion chamber, increasing efficiency. It also produces desirable combustion qualities.
Aside from its use in combustibles, octane is also used as an industrial solvent, lacquer diluent, and carrier solvent in polymer manufacturing. Due to its large variety of uses, Octane is frequently partially responsible for many every day items.
The molecule itself consists of a line of eight carbon molecules, with each carbon having two hydrogens bonded to it (the carbon molecules the ends have three instead of two.) It usually exists as a colorless liquid with an odor of gasoline; it also floats on water.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org