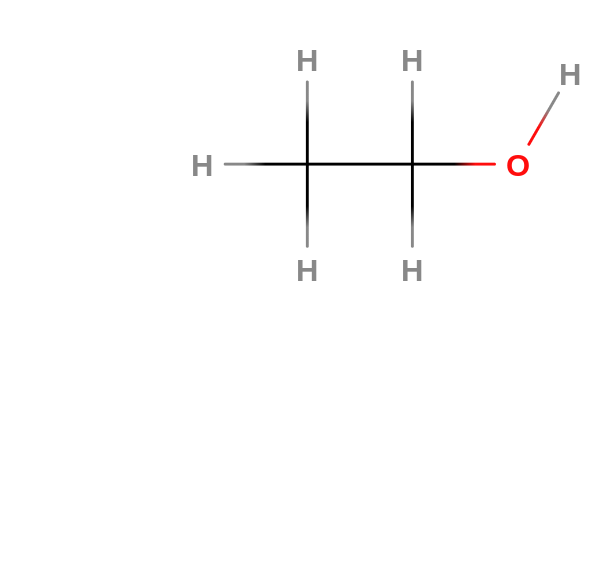
Pure Ethanol is flammable and a colorless liquid. Its low melting point of -144.5° C allows it to be used in antifreeze products. It has a pleasant odor reminiscent of whiskey. It is also easily soluble in water. Ethanol can lose a proton from the hydroxyl group and is a very weak acid, weaker than water. Ethanol is a 2-carbon alcohol. Its molecular formula is CH3CH2OH. Ethanol reduces greenhouse gas emissions by 40-45 percent compared to gasoline. It is renewable fuel because it is produced from biomass. Ethanol also burns more cleanly and completely than gasoline or diesel fuel. Currently only light-duty vehicles with the model year of 2001 or newer use E15 a type of Ethanol.
It is used all around the world but together the U.S.A and Brazil produce 84% of the world’s ethanol.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org