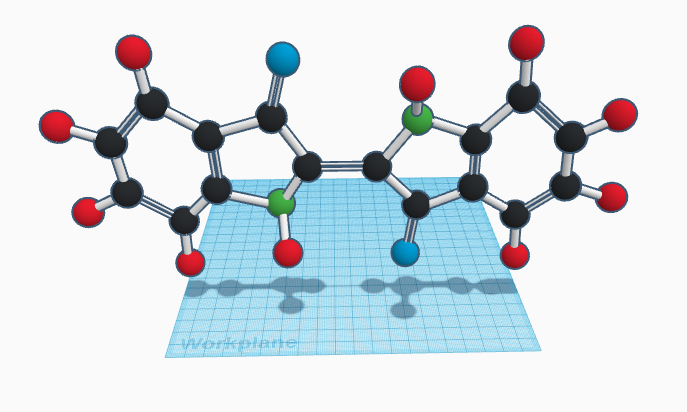
Indigo dye dates back to around 6,000 years ago in Huaca Prieta, Peru. Indigo is a natural dye historically extracted from the leaves of plants in the indigofera genus, although now it is produced synthetically. The deep blue color is caused by the benzene rings—they absorb light and are in many colored molecules. Indigo is used to dye wool, silk, and cotton yarn, as well as being a food dye, but its most common usage is in the very popular denim material.
The chemical formula of indigo is C16H10N2O2. It has two benzene rings and is mostly flat except for two hydrogen-nitrogen bonds, which are set at an angle. Indigo is a partially polar molecule. Twice, a hydrogen is bound to a nitrogen, creating a hydrogen bond and a lot of polarity. There are also two dipole-dipole interactions, when an oxygen atom is bound to a carbon atom.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org