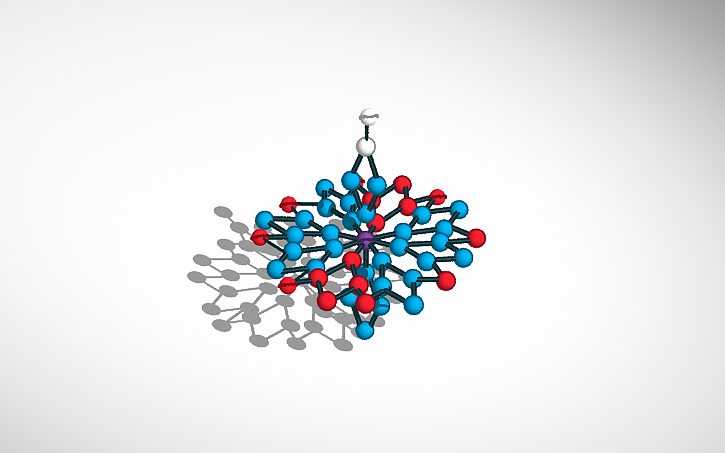
I hate the feeling of grease on my hands! One day while baking, I started wondering if washing my hands with both soap and water was necessary? The answer — Water has a lot of surface tension, which is what allows certain bugs that are denser than the water, to float on it. So cleaning our hands, the surface tension of the water needs to be reduced. That’s where surfactant comes into play. Surfactant decreases the surface tension of the water that we dissolved it in. Sodium Palmate is a type of surfactant that is one of the key components in certain soap brands. It has a chemical formula of C16H31NaO2, meaning it has sixteen carbon molecules, thirty-one hydrogen molecules, one sodium molecule, and two oxygen molecules. Sodium palmate comes from an acid that is derived from palm oil. Sodium Palmate is especially important during these times to be safe.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org