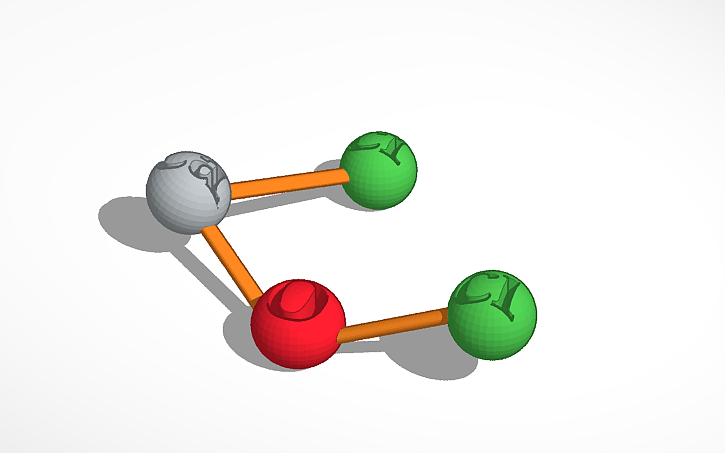
Introduced in 1799 by Scottish chemist Charles Tennant, bleaching powder is a solid, white powder. The smell of this molecule is similar to the odor of chlorine. The scientific name of bleaching powder is Calcium hypochlorite. Bleaching powder is a mixture of calcium hypochlorite and calcium chloride, with the action of chlorine on calcium hydroxide. This molecule is used as a strong disinfectant and to bleach or clean clothes. Bleaching powder can be added to pool water in tablet form to kill germs. The chemical formula of this powerful disinfectant is Ca(ClO)2. Bleaching powder is made up of 1 calcium atom, 1 oxygen atom, and 2 chlorine atoms. The calcium atom is connected to the oxygen atom and one chlorine atom. The other chlorine atom is connected to the oxygen atom. All of the connections are single bonds.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org