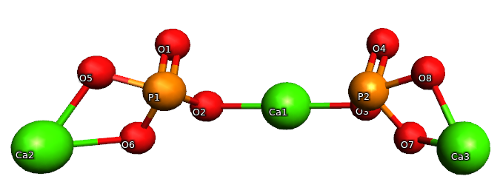
The atom I’m using in this project is called calcium phosphate. Calcium phosphate’s molecule compound is Ca 3 (PO 4) 2. The chemical properties in this equation is, the Ca means calcium and the 3 means times three, the P means phosphate but because it’s in the parentheses with a 2 it means times 2, the O means oxygen and because of the 4 and the 2 it is 8. The number of atoms in calcium phosphate is 13 atoms, and the number of elements is 3. Calcium phosphate’s relevance in the real world contains different types of properties. For example, we use calcium phosphate thicken, stabilize and firm foods, we use it to help keep bones strong, be able to be put in vegan foods, and is used to prevent and treat calcium deficiency. All of these examples of calcium phosphate’s different properties and the things we use everyday.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org