Name the Molecule “Description/Communication”
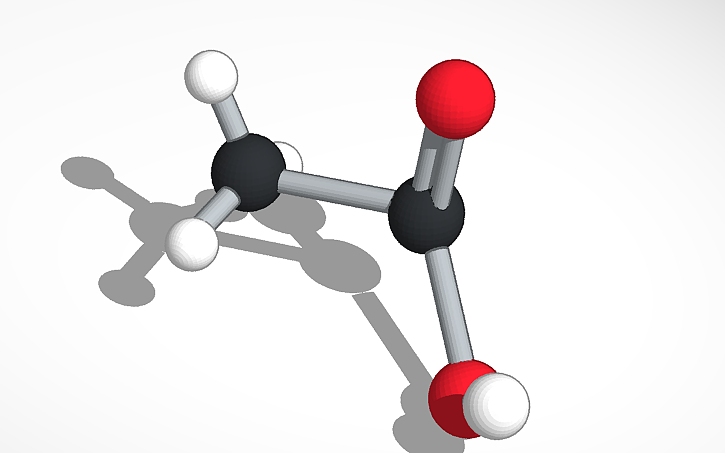
Acetic Acid, the main compound of vinegar, is a clear natural liquid with a strong odor. Its chemical formula is CH3COOH. It has a tart sour taste and it’s miscible in water. Its density is 1.05 g/㎤; the boiling point is 117.9°C; and the melting point is 16.6°C. This compound contains two carbon atoms, four hydrogen atoms and two oxygen atoms. Pure acetic acid is harmful when it contacts skin, but it is safe when it is a dilute solution. Acetic acid is mostly known because of its use in vinegar. This chemical compound is mainly a solvent for inks, paints and coatings. It is used in manufacturing rubber, perfumes, etc. It’s also used in antiseptic creams and injections to treat cancer. All in all, acetic acid (CH3COOH) is a colorless chemical compound that can be used to make many things.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org