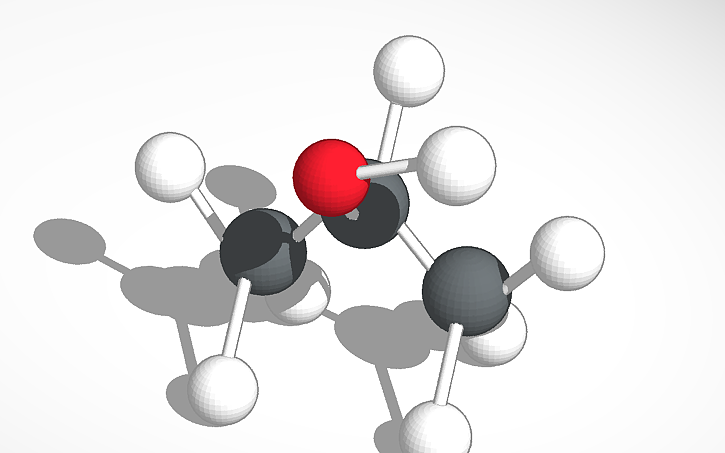
Isopropyl alcohol, which is more commonly known as rubbing alcohol, has a chemical formula of C3H8O, which means that it is made up of three carbon atoms, eight hydrogen atoms, and one oxygen atom. The boiling point of this compound is 180.5°F, and it has a freezing point of -128.2°F. The density of this substance is 786 kg/m³, and it has a molar mass of 60.1 g/mol. Isopropyl alcohol is soluble in substances such as water, ethanol, ether, and chloroform, and it is flammable and low in viscosity. It is volatile, colorless, and has a strong odor. It can be used as an antiseptic for wounds or as a disinfectant. This substance can also be found in items such as alcohol swabs, stain removers, antifreezes, hand sanitizers, and it is used in cosmetics and personal care products.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org