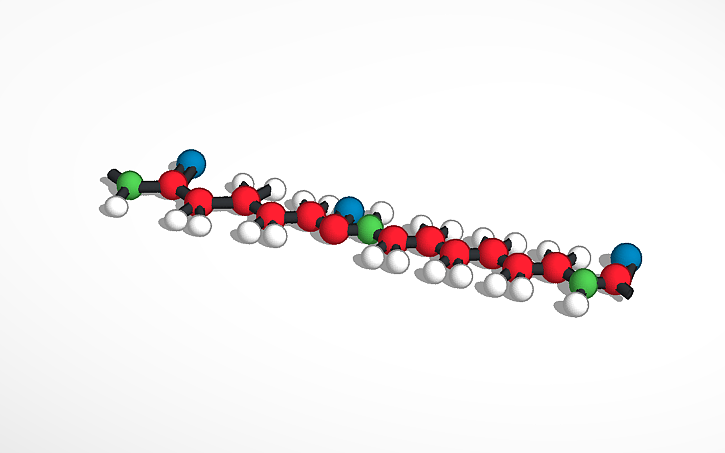
The compound portrayed in the model is called Polyamide, more commonly known as Nylon 66. Nylon 66 is a compound used in everyday life, which could be found in clothing, used in industrial companies, fishnets, and also used as plastic for manufacturing machine parts. Of course for it to be used as such it has to have compatible properties. Some of its properties of course are that it has a melting point of 452.1C or 845.8F. Nylon 66 also has a density of 1.14 g/cm3. A couple more properties of nylon 66 are that it has high mechanical strength and stiffness. There are many other properties out there. Now it’s what actually makes the compound called nylon 66. Nylon 66 is made up of 12 carbon, 22 hydrogens, 2 nitrogen, and 2 oxygen molecules. Make the formula that is written like this (C12H22N2O2).
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org