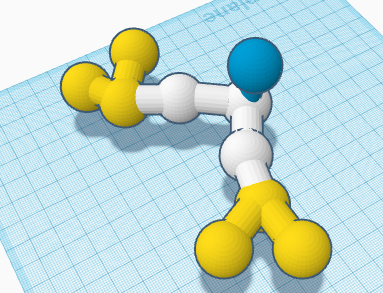
The nailpolish remover is a yellow clear color. It is made up of Carbon, Hydrogen, and Oxygen. There are a total of 10 atoms. 3 Carbon, 6 Hydrogen, and 1 Oxygen. The smell of the nailpolish remover is of acetone which is a ketone. It has a low viscosity. When shaking the nailpolish remover the bubbles would start at the bottom and rise up to the top. As soon as the bottle is placed down the bubbles start to pop. It is extremely flammable and will ignite on fire if it comes too close to a flame. It can be harmful to fabrics and is dangerously poisonous. It has a boiling point of 56.2 degrees celsius. It has a density of 0.7857 g/cm3. It has a melting point of 95 degrees celsius. The nailpolish remover is soluble in water. The scientific name is Acetone which is also the compound.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org