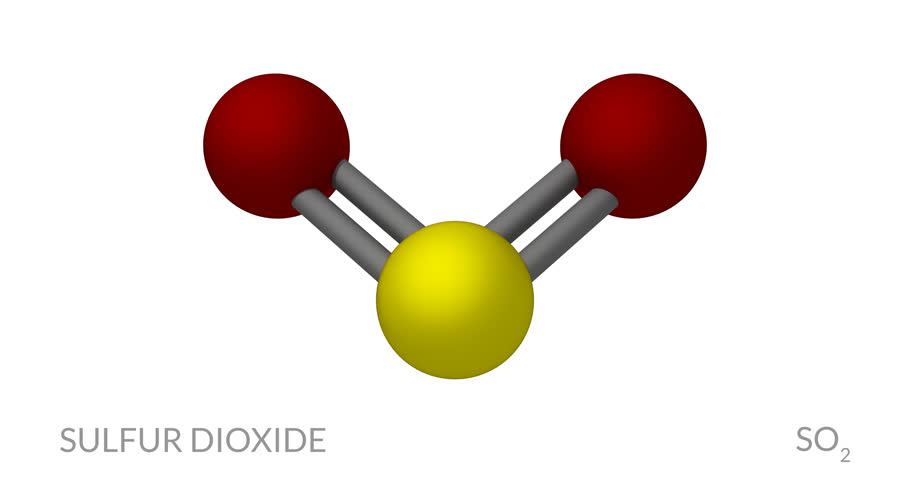
Sulfur Dioxide is a molecule that is bent with polar covalent bonds between one sulfur atom and two oxygen atoms. It is also colorless water-soluble gas that forms when sulfur burns. Sulfur Dioxide is not flammable but can turn into Sulfur Trioxide when it is exposed to Oxygen. The chemical formula is SO2. It is poisonous and toxic. The molar mass is 64.066 g/mol. Sulfur Dioxide is many of which are called pollutants in the atmosphere. The atomic number is 16. The atomic weight is 32.065. The number of isotopes is 23. Sulfur Dioxide’s melting point is 239.38 degrees Fahrenheit and the boiling point is 832.28 degrees Fahrenheit.
Download File
Download File
Contact us
Thank you for your interest in contacting Future Engineers. We look forward to connecting with you!
General Inquiries
support@futureengineers.orgSponsorship Inquiries
sponsor@futureengineers.org